What is the average atomic weight for a hypothetical element "Q" which is composed of the following mass isotopes and their corresponding percent mass abundances? Hint: express your answer to four significant figures or risk losing all credit! Do not include the unit "amu" in your answer. mass of % mass isotope abundance (amu) 39.63 32. 11 40.59 55.24 42.99 12.65
What is the average atomic weight for a hypothetical element "Q" which is composed of the following mass isotopes and their corresponding percent mass abundances? Hint: express your answer to four significant figures or risk losing all credit! Do not include the unit "amu" in your answer. mass of % mass isotope abundance (amu) 39.63 32. 11 40.59 55.24 42.99 12.65
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 21PS: Verify that the atomic weight of lithium is 6.94, given the following information: 6Li, mass =...
Related questions
Question
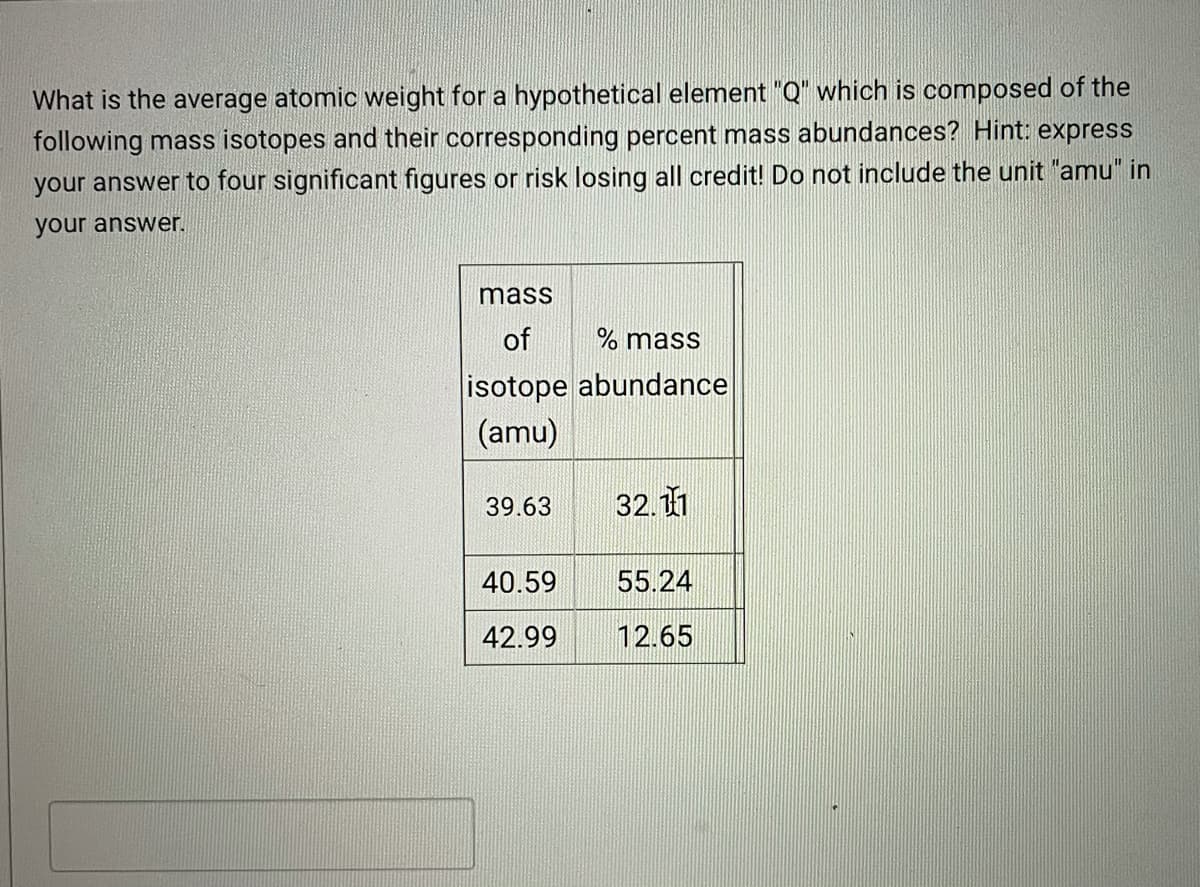
Transcribed Image Text:What is the average atomic weight for a hypothetical element "Q" which is composed of the
following mass isotopes and their corresponding percent mass abundances? Hint: express
your answer to four significant figures or risk losing all credit! Do not include the unit "amu" in
your answer.
mass
of
% mass
isotope abundance
(amu)
39.63
32.11
40.59
55.24
42.99
12.65
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
