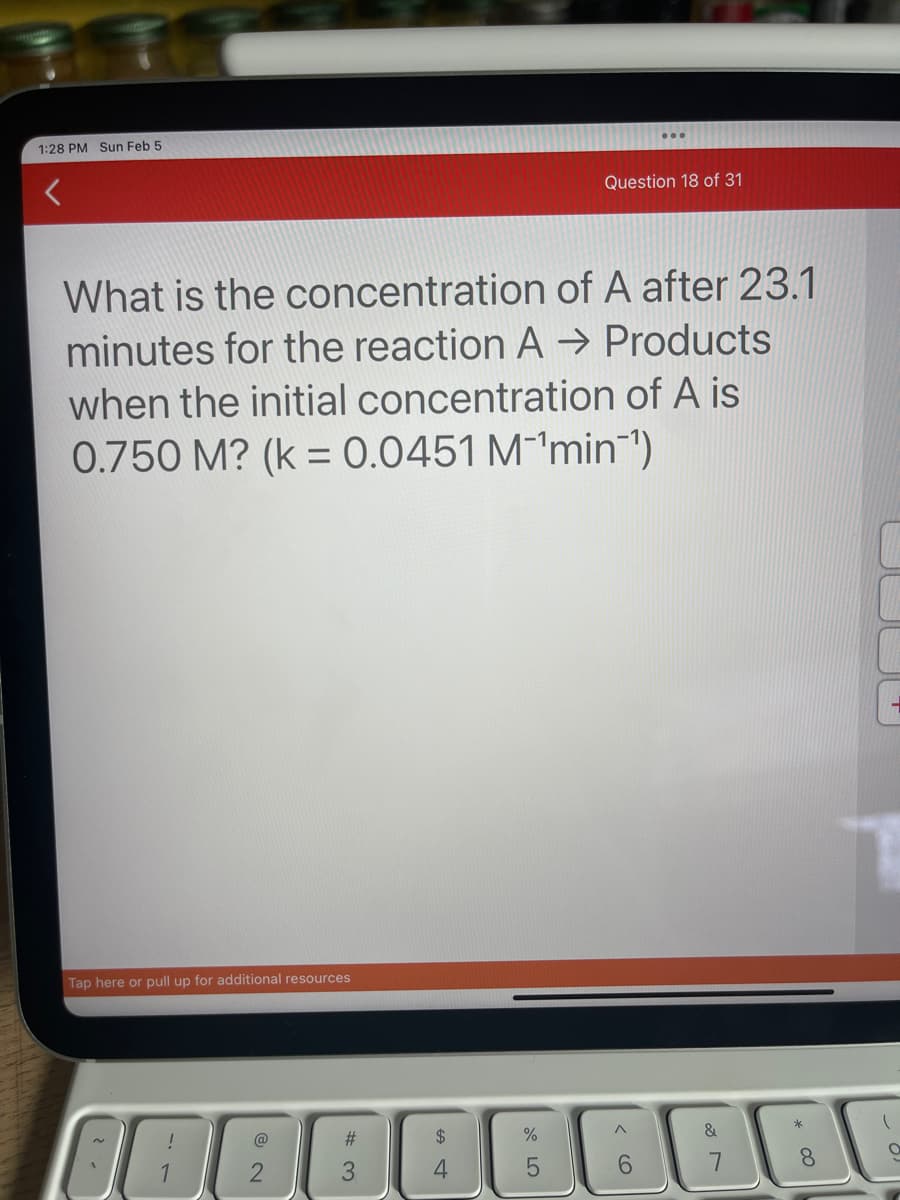
What is the concentration of A after 23.1 minutes for the reaction A → Products when the initial concentration of A is 0.750 M? (k = 0.0451 M¹min¯¹)
What is the concentration of A after 23.1 minutes for the reaction A → Products when the initial concentration of A is 0.750 M? (k = 0.0451 M¹min¯¹)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 22QAP: . Suppose that for the reaction 2N2O(g)+O2(g)4NO((g))it is determined, at a particular temperature,...
Related questions
Question
100%
Transcribed Image Text:1:28 PM Sun Feb 5
What is the concentration of A after 23.1
minutes for the reaction A → Products
when the initial concentration of A is
0.750 M? (k = 0.0451 M¹min¯¹)
Tap here or pull up for additional resources
!
1
@
#
3
Question 18 of 31
%
6
&
*00
8
T
O |
Expert Solution
Step 1
By identifying the order of reaction and using integrated rate equation we can identify the concentration of A after 23.1 minutes.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
