Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:Elaine N. Marieb, Katja N. Hoehn
Chapter1: The Human Body: An Orientation
Section: Chapter Questions
Problem 1RQ: The correct sequence of levels forming the structural hierarchy is A. (a) organ, organ system,...
Related questions
Question
What is the cost of sexual reproduction for males of Teleogryllus oceanicus on Kauai island?

Transcribed Image Text:biology
letters
Biol. Lett. (2006) 2, 521-524
doi:10.1098/rsbl.2006.0539
Published online 19 September 2006
Silent night: adaptive
disappearance of a sexual
signal in a parasitized
population of field crickets
Marlene Zuk*, John T. Rotenberry
and Robin M. Tinghitella
Department of Biology, University of California, Riverside,
CA 92521, USA
* Author for correspondence (marlene.zuk@ucr.edu).
Sexual signa re often critical for mat attrac-
tion and reproduction, although their conspicu-
ousness exposes them to parasites and
predators. We document the near-disappearance
of song, the sexual signal of crickets, and its
replacement with a novel silent morph, in a
population subject to strong natural selection by
a deadly acoustically orienting parasitoid fly. On
the Hawaiian Island of Kauai, more than 90% of
male field crickets (Teleogryllus oceanicus)
shifted in less than 20 generations from a
normal-wing morphology to a mutated wing
that renders males unable to call (flatwing).
Flatwing morphology protects male crickets
from the parasitoid, which uses song to find
hosts, but poses obstacles for mate attraction,
since females also use the males' song to locate
mates. Field experiments support the hypothesis
that flatwings overcome the difficulty of attract-
ing females without song by acting as ‘satellites’
to the few remaining callers, showing enhanced
phonotaxis to the calling song that increases
female encounter rate. Thus, variation in
behaviour facilitated establishment of an other-
wise maladaptive morphological mutation.
Keywords: phonotactic parasitoid; rapid evolution;
satellite
1. INTRODUCTION
Sexual signals such as colourful plumage are critical
for mate attraction and hence reproduction, even
though their conspicuousness exposes them to
parasites and predators (Zuk & Kolluru 1998). Such
signals often represent compromises between natural
and sexual selection. Since 1991, we have been
examining the responses to such conflicting selective
pressure in populations of the field cricket Teleogryllus
oceanicus, an Australian and Pacific Island species
introduced to three Hawaiian Islands (Oahu, the Big
Island of Hawaii and Kauai), where it is subject to an
acoustically orienting parasitoid fly, Ormia ochracea
(Zuk et al. 1993). The parasitoid is North American
in origin and overlaps in range with T. oceanicus only
in Hawaii (Lehmann 2003). The fly finds its host
using the same signal (the calling song) that males
produce to attract mates; fly larvae burrow into the
The electronic supplementary material is available at http://dx.doi.
org/10.1098/rsbl.2006.0539 or via http://www.journals.royalsoc.ac.
uk.
cricket and develop inside, killing the host upon
emergence.
Previous work demonstrated that parasitized popu-
lations have altered song structure, response to
disturbance and calling behaviour compared with
unparasitized populations (Zuk et al. 1993, 1995,
1998, 2001; Rotenberry et al. 1996; Lewkiewicz &
Zuk 2004). Here we document a much more extreme
and rapid adaptive change, near-complete loss of
calling, in the Kauai population, and examine its
consequences for mate location and the evolution of
mate choice in the context of interaction between
behavioural plasticity and morphological adaptation.
Kauai has always had the highest prevalence of the
parasitoid, with nearly 30% of calling males harbour-
ing the fly (Zuk et al. 1993). Presumably owing to the
associated mortality, with each field visit since 1991
we heard and observed fewer crickets on that island,
and in 2001 only heard a single calling male, with all
crickets extremely scarce in intensive searches
(methods; see electronic supplementary material).
Over a three day visit in 2003, although we heard
none calling, crickets were far more abundant than
before in their habitat of fields and lawns. Further
examination revealed that virtually all Kauai males
had female-like wings, lacking the normal stridulatory
apparatus of file and scraper required for sound
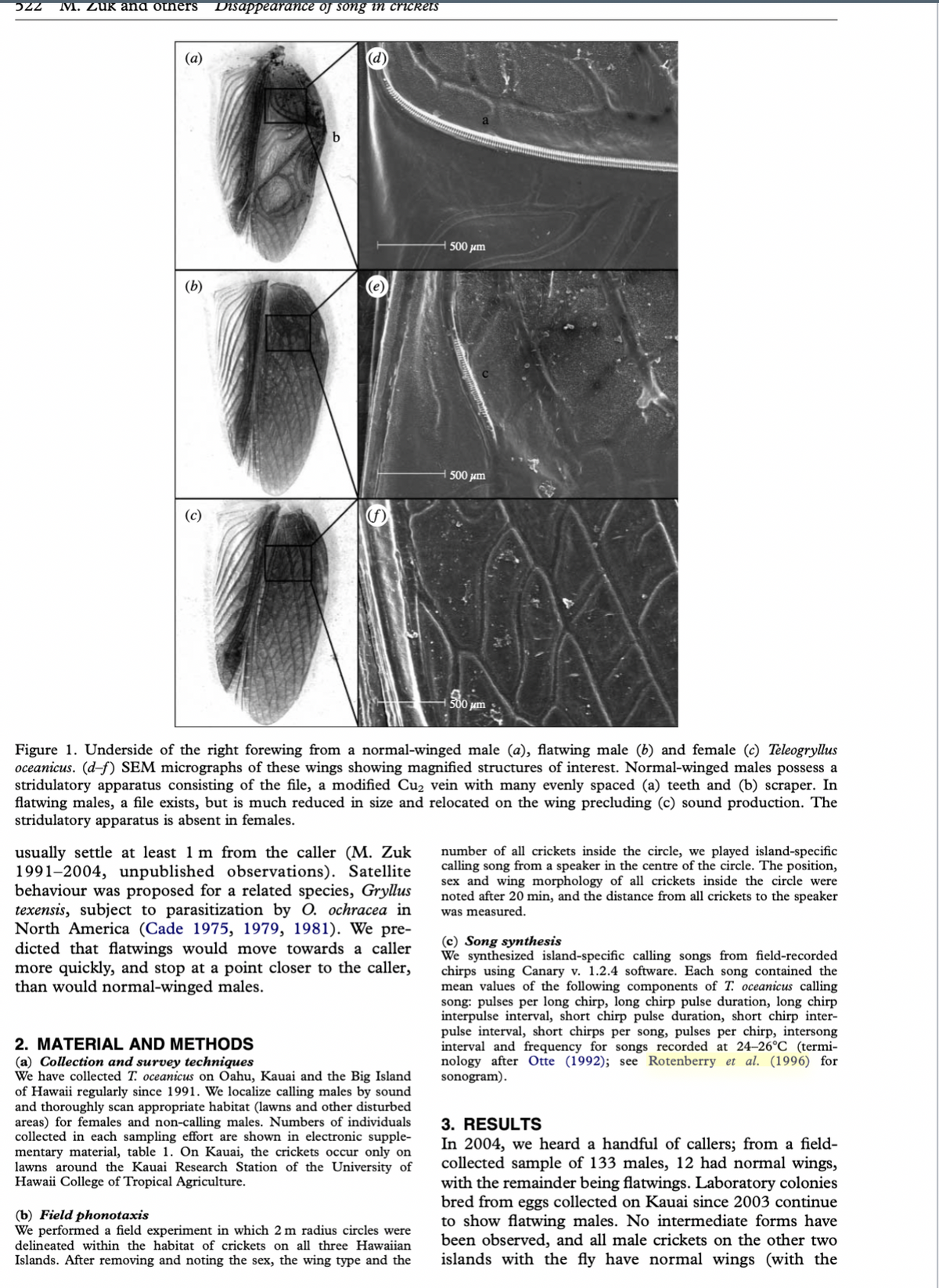
production (hereafter called flatwings; figure 1).
Instead, the file is reduced in size and relocated at an
angle precluding sound production (figure 1). Flatw-
ings are thus unable to call. Populations from the
other Hawaiian Islands as well as descendents from
eggs collected on Kauai before 2003 continue to
exhibit normal wings.
Loss of calling clearly protects the crickets from
the parasitoid. Although flies are still attracted to
sound traps on Kauai, out of 121 flatwings dissected,
only one harboured parasitoid larvae, versus greater
than 30% infestation rates previously associated with
normal-winged males on Kauai. But this protection
comes with the price of losing the sexual signal. How
do females locate silent flatwing males? Moreover,
like most field crickets, T. oceanicus males produce a
courtship song after a female is within close range.
Females in this and other species require the male to
produce the courtship song before mounting to
receive a spermatophore (Burk 1983; Libersat et al.
1994). Flatwings can produce neither calling nor
courtship song, and crickets are not known to use
long-range pheromones for mate location (Tregenza &
Wedell 1997). Nevertheless, the now-thriving popu-
lation of T. oceanicus on Kauai suggests that the
obstacles in both detecting and accepting mates have
been overcome.
Here, we focus on the difficulty of long-range mate
location. We propose that flatwing male T. oceanicus
on Kauai behave as 'satellites' (Cade 1980) to the few
calling males that remain, with an enhanced phono-
taxis response to a calling song that brings them into
close proximity with the caller. Attracted females
should then be much more likely to encounter
flatwings as potential mates than they would if the
flatwings simply moved at random throughout the
habitat. Male crickets from a variety of species,
including T. oceanicus, are normally attracted to the
song of other males (Kiflawi & Gray 2000), but they
Transcribed Image Text:OZZ M. Zuk and others
(a)
(b)
(c)
Disappearance of song in crickets
b
(d)
usually settle at least 1 m from the caller (M. Zuk
1991-2004, unpublished observations). Satellite
behaviour was proposed for a related species, Gryllus
texensis, subject to parasitization by O. ochracea in
North America (Cade 1975, 1979, 1981). We pre-
dicted that flatwings would move towards a caller
more quickly, and stop at a point closer to the caller,
than would normal-winged males.
Figure 1. Underside of the right forewing from a normal-winged male (a), flatwing male (b) and female (c) Teleogryllus
oceanicus. (d-f) SEM micrographs of these wings showing magnified structures of interest. Normal-winged males possess a
stridulatory apparatus consisting of the file, a modified Cu₂ vein with many evenly spaced (a) teeth and (b) scraper. In
flatwing males, a file exists, but is much reduced in size and relocated on the wing precluding (c) sound production. The
stridulatory apparatus is absent in females.
2. MATERIAL AND METHODS
(a) Collection and survey techniques
We have collected T. oceanicus on Oahu, Kauai and the Big Island
of Hawaii regularly since 1991. We localize calling males by sound
and thoroughly scan appropriate habitat (lawns and other disturbed
areas) for females and non-calling males. Numbers of individuals
collected in each sampling effort are shown in electronic supple-
mentary material, table 1. On Kauai, the crickets occur only on
lawns around the Kauai Research Station of the University of
Hawaii College of Tropical Agriculture.
500 μm
(b) Field phonotaxis
We performed a field experiment in which 2 m radius circles were
delineated within the habitat of crickets on all three Hawaiian
Islands. After removing and noting the sex, the wing type and the
500 μm
number of all crickets inside the circle, we played island-specific
calling song from a speaker in the centre of the circle. The position,
sex and wing morphology of all crickets inside the circle were
noted after 20 min, and the distance from all crickets to the speaker
was measured.
(c) Song synthesis
We synthesized island-specific calling songs from field-recorded
chirps using Canary v. 1.2.4 software. Each song contained the
mean values of the following components of T. oceanicus calling
song: pulses per long chirp, long chirp pulse duration, long chirp
interpulse interval, short chirp pulse duration, short chirp inter-
pulse interval, short chirps per song, pulses per chirp, intersong
interval and frequency for songs recorded at 24-26°C (termi-
nology after Otte (1992); see Rotenberry et al. (1996) for
sonogram).
3. RESULTS
In 2004, we heard a handful of callers; from a field-
collected sample of 133 males, 12 had normal wings,
with the remainder being flatwings. Laboratory colonies
bred from eggs collected on Kauai since 2003 continue
to show flatwing males. No intermediate forms have
been observed, and all male crickets on the other two
islands with the fly have normal wings (with the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:
9780134580999
Author:
Elaine N. Marieb, Katja N. Hoehn
Publisher:
PEARSON

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781259398629
Author:
McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:
Mcgraw Hill Education,

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:
9780134580999
Author:
Elaine N. Marieb, Katja N. Hoehn
Publisher:
PEARSON

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781259398629
Author:
McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:
Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:
9780815344322
Author:
Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:
W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:
9781260159363
Author:
Martin, Terry R., Prentice-craver, Cynthia
Publisher:
McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:
9781260231700
Author:
Sylvia S. Mader, Michael Windelspecht
Publisher:
McGraw Hill Education