Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter25: Catalytic Hydrogenation
Section: Chapter Questions
Problem 6Q
Related questions
Question
C.8.
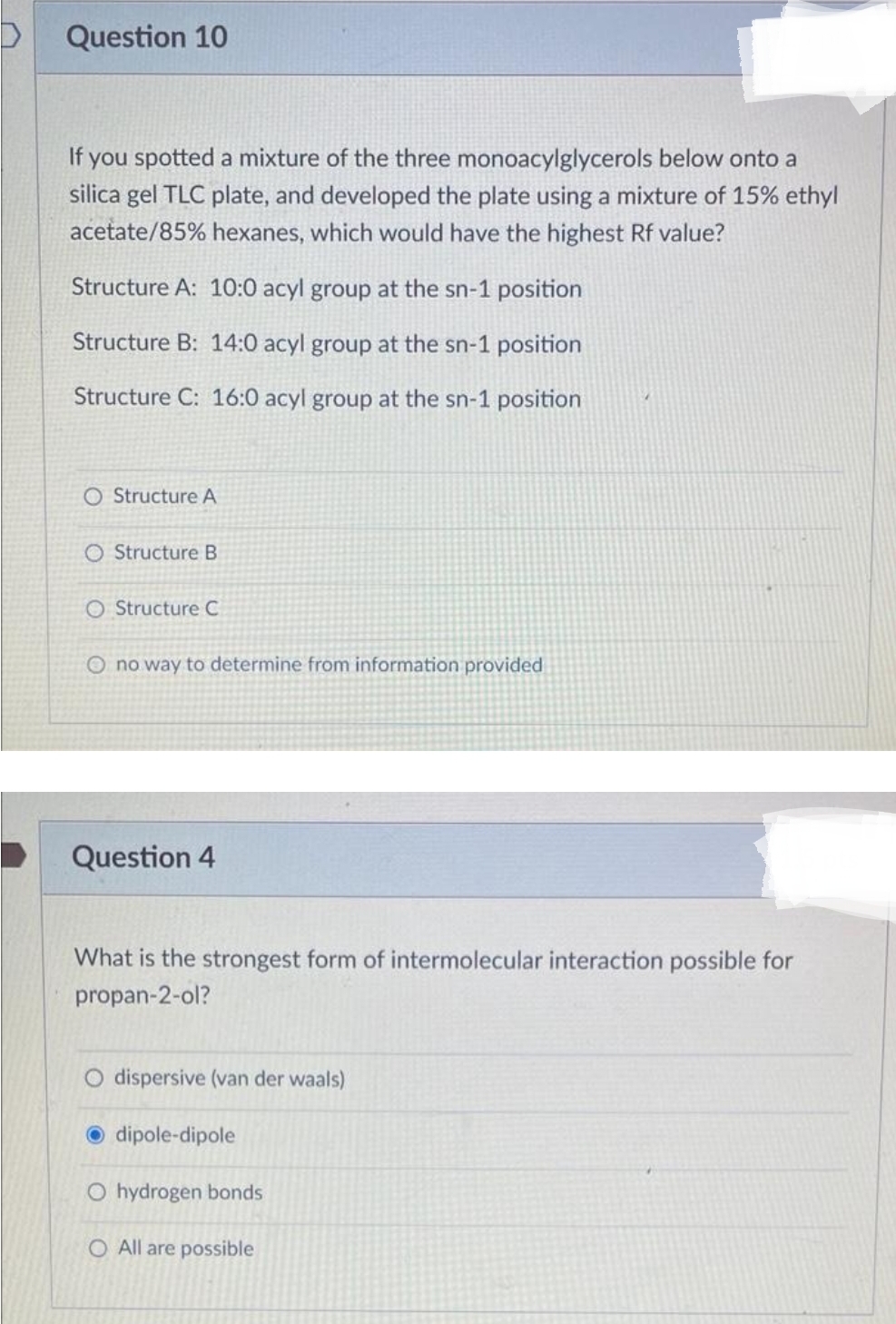
Transcribed Image Text:D
Question 10
If you spotted a mixture of the three monoacylglycerols below onto a
silica gel TLC plate, and developed the plate using a mixture of 15% ethyl
acetate/85% hexanes, which would have the highest Rf value?
Structure A: 10:0 acyl group at the sn-1 position
Structure B: 14:0 acyl group at the sn-1 position
Structure C: 16:0 acyl group at the sn-1 position
O Structure A
O Structure B
O Structure C
O no way to determine from information provided
Question 4
What is the strongest form of intermolecular interaction possible for
propan-2-ol?
O dispersive (van der waals)
dipole-dipole
O hydrogen bonds
O All are possible
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning