What is the value of the principal quantum number, n, for the hydrogen s orbital matching these curves? n= How many nodes are predicted by the graph? Radial wave function nodes 4 10 15 0 2530 35 40 45 50 55 60 65 70 75 80 Radius (A) According to the graph, select the appropriate x-axis values for the location of nodes in terms of the Bohr radii Here is an expanded view of just (0.529 Å) the portion of the graph from x 0 to x 8 39.5 27.0 3 4 5 6.4 3.9 10.1 0.7 1.9 13.9 20.7
What is the value of the principal quantum number, n, for the hydrogen s orbital matching these curves? n= How many nodes are predicted by the graph? Radial wave function nodes 4 10 15 0 2530 35 40 45 50 55 60 65 70 75 80 Radius (A) According to the graph, select the appropriate x-axis values for the location of nodes in terms of the Bohr radii Here is an expanded view of just (0.529 Å) the portion of the graph from x 0 to x 8 39.5 27.0 3 4 5 6.4 3.9 10.1 0.7 1.9 13.9 20.7
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 10P: (a) Use the radial wave function for the 3p orbital of a hydrogen atom (see Table 5.2) to calculate...
Related questions
Question
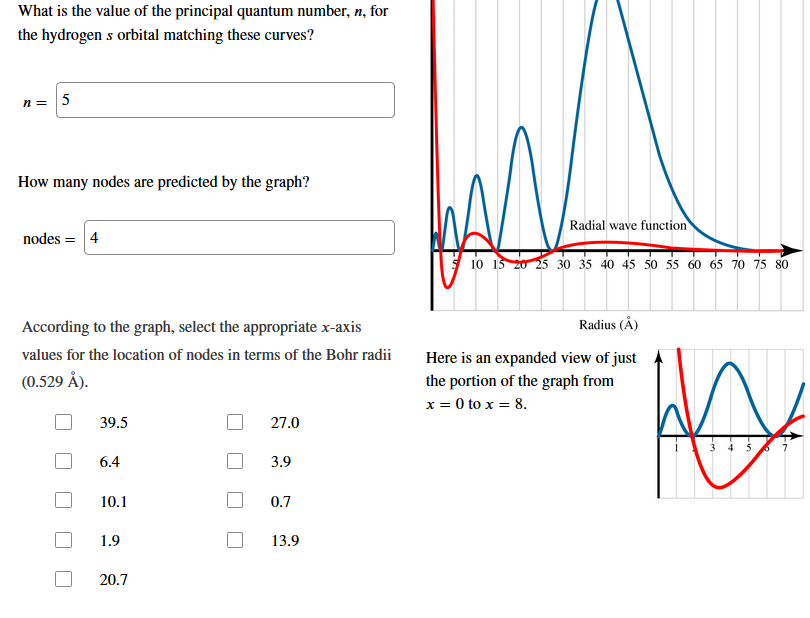
Transcribed Image Text:What is the value of the principal quantum number, n, for
the hydrogen s orbital matching these curves?
n=
How many nodes are predicted by the graph?
Radial wave function
nodes
4
10 15 0 2530 35 40 45 50 55 60 65 70 75 80
Radius (A)
According to the graph, select the appropriate x-axis
values for the location of nodes in terms of the Bohr radii
Here is an expanded view of just
(0.529 Å)
the portion of the graph from
x 0 to x 8
39.5
27.0
3 4 5
6.4
3.9
10.1
0.7
1.9
13.9
20.7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning