What volume of 0.305 M AgNO3 is required to react exactly with 155.0 mL of 0.274 M Na2SO4 solution? Hint: you will want to write a balanced reaction. 139 mL 278 mL 581 mL 345 mL 173 mL
What volume of 0.305 M AgNO3 is required to react exactly with 155.0 mL of 0.274 M Na2SO4 solution? Hint: you will want to write a balanced reaction. 139 mL 278 mL 581 mL 345 mL 173 mL
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.16QAP
Related questions
Question
100%
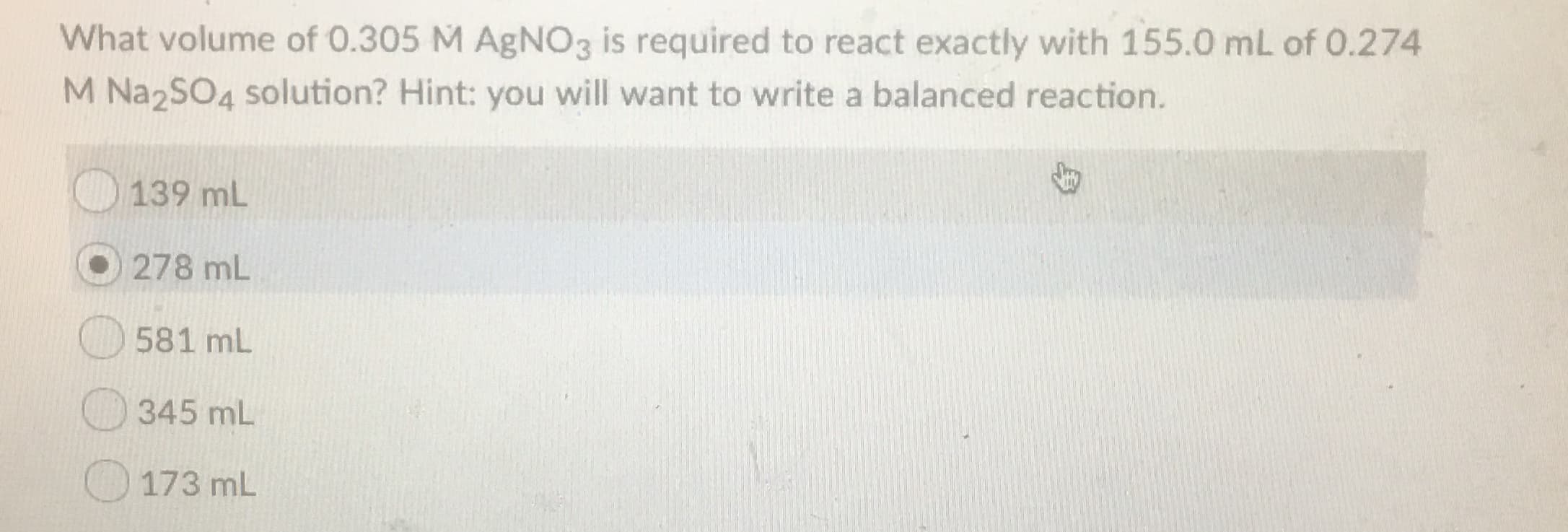
Transcribed Image Text:What volume of 0.305 M AgNO3 is required to react exactly with 155.0 mL of 0.274
M Na2SO4 solution? Hint: you will want to write a balanced reaction.
139 mL
278 mL
581 mL
345 mL
173 mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
