When considering the effects of osmotic pressure, we assumed that the mem- brane was completely impermeable to the solute. In real life, membranes can be partially permeable to a solute. This situation can be described by a slightly modified form of Equation (4.64) Q12 = ALp(Ap – oAn) where Q12 is the fluid flow rate across the membrane, A is the membrane area, L, is the membrane permeability, Ap is the pressure difference across (4.90) the membrane, o is the osmotic reflection coefficient for the solute, and An is the osmotic pressure difference across the membrane.16 The new feature here is the reflection coefficient, o , which depends on the molecular weight (size) of the solute. Note that o = 1 for a perfectly rejected solute and o = 0 for a freely permeable solute. (a) Suppose that the solute is spherical. Show by a simple proportionality argument that the effective radius of the solute, r,, should vary as r, ~ (MW)!/3, where MW is the molecular weight of the solute. (b) Consider Lp, A, and Ap to be constants, and suppose a fixed mass of solute is added to the water on the right side of the membrane in Fig. 4.42. Argue that there must be a certain solute size, r, that max- imizes Q12. You do not need any mathematics for this part of the question; a written argument is sufficient. Hint: consider two limiting cases: a very small solute particle and a very large one. Think about what happens to the molar concentration as the MW gets large for a fixed mass of solute. (c) When the solute radius is close to the membrane pore radius, rp, Ferry [56] showed that the reflection coefficient varies as o =1 -2(1 – n)? – (1 – n)“, where n = rs/rp, for n < 1. Show that in this case, the maximum Q12 occurs for a solute radius rs/r, = 2 – (5/2)/2. You may assume van’t Hoff's law holds for the solute and neglect the fourth-order term in the expression for o. P2 1) P1 P2 P1 Q12 T2 Water + solute Water Membrane Figure 4.42
When considering the effects of osmotic pressure, we assumed that the mem- brane was completely impermeable to the solute. In real life, membranes can be partially permeable to a solute. This situation can be described by a slightly modified form of Equation (4.64) Q12 = ALp(Ap – oAn) where Q12 is the fluid flow rate across the membrane, A is the membrane area, L, is the membrane permeability, Ap is the pressure difference across (4.90) the membrane, o is the osmotic reflection coefficient for the solute, and An is the osmotic pressure difference across the membrane.16 The new feature here is the reflection coefficient, o , which depends on the molecular weight (size) of the solute. Note that o = 1 for a perfectly rejected solute and o = 0 for a freely permeable solute. (a) Suppose that the solute is spherical. Show by a simple proportionality argument that the effective radius of the solute, r,, should vary as r, ~ (MW)!/3, where MW is the molecular weight of the solute. (b) Consider Lp, A, and Ap to be constants, and suppose a fixed mass of solute is added to the water on the right side of the membrane in Fig. 4.42. Argue that there must be a certain solute size, r, that max- imizes Q12. You do not need any mathematics for this part of the question; a written argument is sufficient. Hint: consider two limiting cases: a very small solute particle and a very large one. Think about what happens to the molar concentration as the MW gets large for a fixed mass of solute. (c) When the solute radius is close to the membrane pore radius, rp, Ferry [56] showed that the reflection coefficient varies as o =1 -2(1 – n)? – (1 – n)“, where n = rs/rp, for n < 1. Show that in this case, the maximum Q12 occurs for a solute radius rs/r, = 2 – (5/2)/2. You may assume van’t Hoff's law holds for the solute and neglect the fourth-order term in the expression for o. P2 1) P1 P2 P1 Q12 T2 Water + solute Water Membrane Figure 4.42
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
![When considering the effects of osmotic pressure, we assumed that the mem-
brane was completely impermeable to the solute. In real life, membranes
can be partially permeable to a solute. This situation can be described by a
slightly modified form of Equation (4.64)
Q12 = ALp(Ap – oAn)
where Q12 is the fluid flow rate across the membrane, A is the membrane
area, L, is the membrane permeability, Ap is the pressure difference across
(4.90)
the membrane, o is the osmotic reflection coefficient for the solute, and An
is the osmotic pressure difference across the membrane.16 The new feature
here is the reflection coefficient, o , which depends on the molecular weight
(size) of the solute. Note that o = 1 for a perfectly rejected solute and o = 0
for a freely permeable solute.
(a) Suppose that the solute is spherical. Show by a simple proportionality
argument that the effective radius of the solute, r,, should vary as
r, ~ (MW)!/3, where MW is the molecular weight of the solute.
(b) Consider Lp, A, and Ap to be constants, and suppose a fixed mass
of solute is added to the water on the right side of the membrane in
Fig. 4.42. Argue that there must be a certain solute size, r, that max-
imizes Q12. You do not need any mathematics for this part of the
question; a written argument is sufficient. Hint: consider two limiting
cases: a very small solute particle and a very large one. Think about
what happens to the molar concentration as the MW gets large for a
fixed mass of solute.
(c) When the solute radius is close to the membrane pore radius,
rp, Ferry [56] showed that the reflection coefficient varies as
o =1 -2(1 – n)? – (1 – n)“, where n = rs/rp, for n < 1. Show
that in this case, the maximum Q12 occurs for a solute radius
rs/r, = 2 – (5/2)/2. You may assume van’t Hoff's law holds for the
solute and neglect the fourth-order term in the expression for o.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Feec3cad0-e4a7-46a3-ae05-d6d01310238d%2F68fd05a5-f033-48f5-9952-ac261fd6603a%2Fppm714.png&w=3840&q=75)
Transcribed Image Text:When considering the effects of osmotic pressure, we assumed that the mem-
brane was completely impermeable to the solute. In real life, membranes
can be partially permeable to a solute. This situation can be described by a
slightly modified form of Equation (4.64)
Q12 = ALp(Ap – oAn)
where Q12 is the fluid flow rate across the membrane, A is the membrane
area, L, is the membrane permeability, Ap is the pressure difference across
(4.90)
the membrane, o is the osmotic reflection coefficient for the solute, and An
is the osmotic pressure difference across the membrane.16 The new feature
here is the reflection coefficient, o , which depends on the molecular weight
(size) of the solute. Note that o = 1 for a perfectly rejected solute and o = 0
for a freely permeable solute.
(a) Suppose that the solute is spherical. Show by a simple proportionality
argument that the effective radius of the solute, r,, should vary as
r, ~ (MW)!/3, where MW is the molecular weight of the solute.
(b) Consider Lp, A, and Ap to be constants, and suppose a fixed mass
of solute is added to the water on the right side of the membrane in
Fig. 4.42. Argue that there must be a certain solute size, r, that max-
imizes Q12. You do not need any mathematics for this part of the
question; a written argument is sufficient. Hint: consider two limiting
cases: a very small solute particle and a very large one. Think about
what happens to the molar concentration as the MW gets large for a
fixed mass of solute.
(c) When the solute radius is close to the membrane pore radius,
rp, Ferry [56] showed that the reflection coefficient varies as
o =1 -2(1 – n)? – (1 – n)“, where n = rs/rp, for n < 1. Show
that in this case, the maximum Q12 occurs for a solute radius
rs/r, = 2 – (5/2)/2. You may assume van’t Hoff's law holds for the
solute and neglect the fourth-order term in the expression for o.
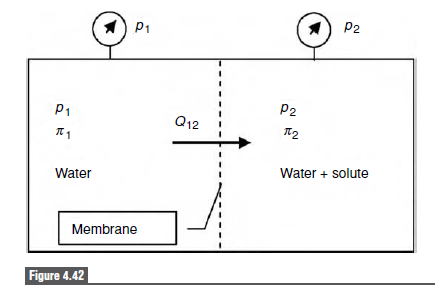
Transcribed Image Text:P2
1) P1
P2
P1
Q12
T2
Water + solute
Water
Membrane
Figure 4.42
Expert Solution
Step 1

Step 2

Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
