When methanol, CH, OH, is burned in the presence of oxygen gas, O,, a large amount of heat energy is released. For this reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced, thermochemical equation CH, OH(g) + 0,(g) CO,(g) + 2 H,0(1) AH = –764 kJ How much methanol, in grams, must be burned to produce 629 kJ of heat? -26.33 mass:
When methanol, CH, OH, is burned in the presence of oxygen gas, O,, a large amount of heat energy is released. For this reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced, thermochemical equation CH, OH(g) + 0,(g) CO,(g) + 2 H,0(1) AH = –764 kJ How much methanol, in grams, must be burned to produce 629 kJ of heat? -26.33 mass:
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 113SCQ
Related questions
Question
Transcribed Image Text:Sign in
Week 15 Recitation
Caleb Young
Oklahoma - CHEM 1315 (Section 001) - Fall20 - ROCHER > Activities and Due Dates > Week 15 Recitation
O Assignment Score:
50%
Resources
O Hint
Check Answer
Question 9 of 10 >
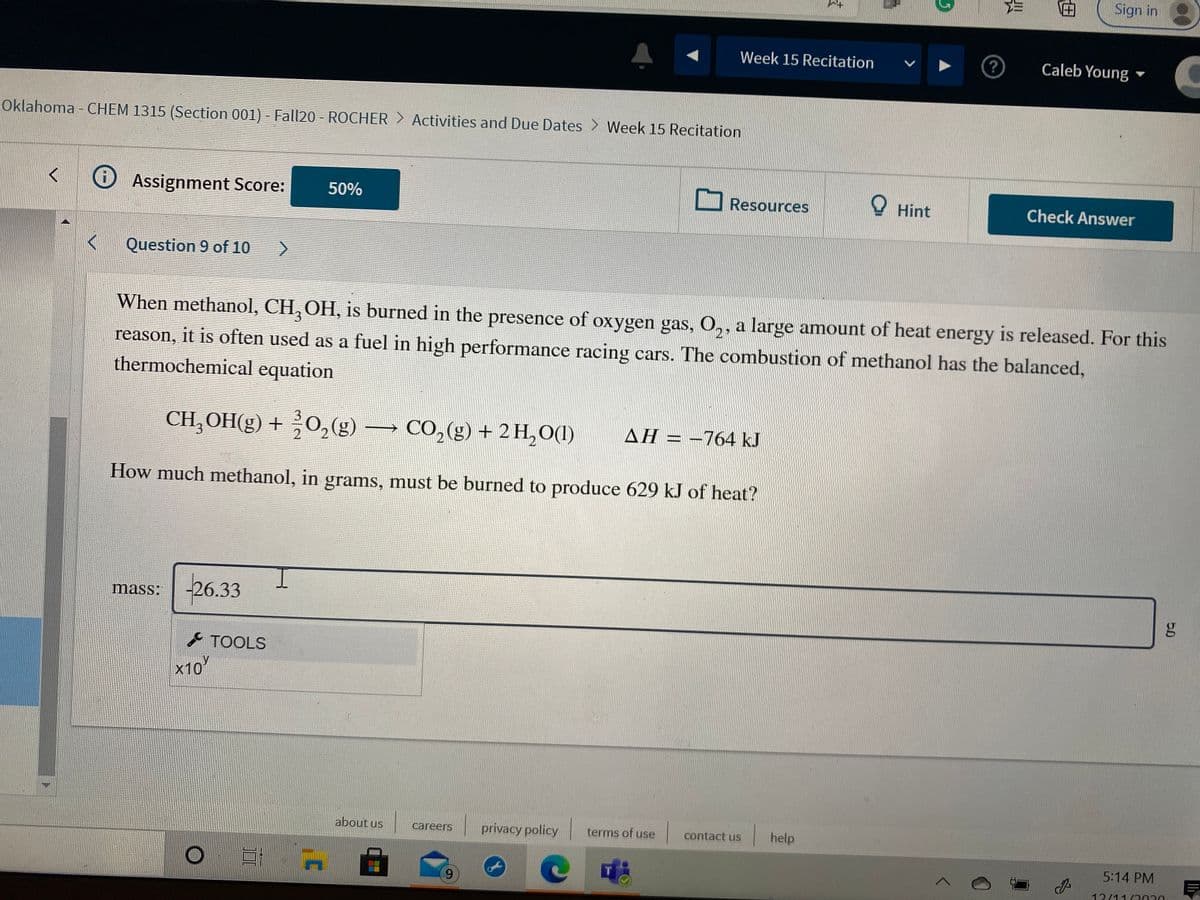
When methanol, CH, OH, is burned in the presence of oxygen gas, O,, a large amount of heat energy is released. For this
2:
reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced,
thermochemical equation
CH,OH(g) + 0,(g) → CO,(g) + 2H,O(1)
AH = –764 kJ
How much methanol, in grams, must be burned to produce 629 kJ of heat?
26.33
mass:
TOOLS
x10
about us
careers
privacy policy
terms of use
contact us
help
5:14 PM
12/11/2020
50
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning