When methanol, CH,OH, is burned in the presence oxygen gas, 0, a large amount of heat energy is released. For this of reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced, thermochemical equation CH,OH(g) + 0,(g) CO,(g) + 2H,0(0) AH = -764 kJ | %3D How much methanol, in grams, must be burned to produce 945 kJ of heat?
When methanol, CH,OH, is burned in the presence oxygen gas, 0, a large amount of heat energy is released. For this of reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced, thermochemical equation CH,OH(g) + 0,(g) CO,(g) + 2H,0(0) AH = -764 kJ | %3D How much methanol, in grams, must be burned to produce 945 kJ of heat?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 65QAP: Natural gas companies in the United States use the therm as a unit of energy. One therm is 1105 BTU....
Related questions
Question
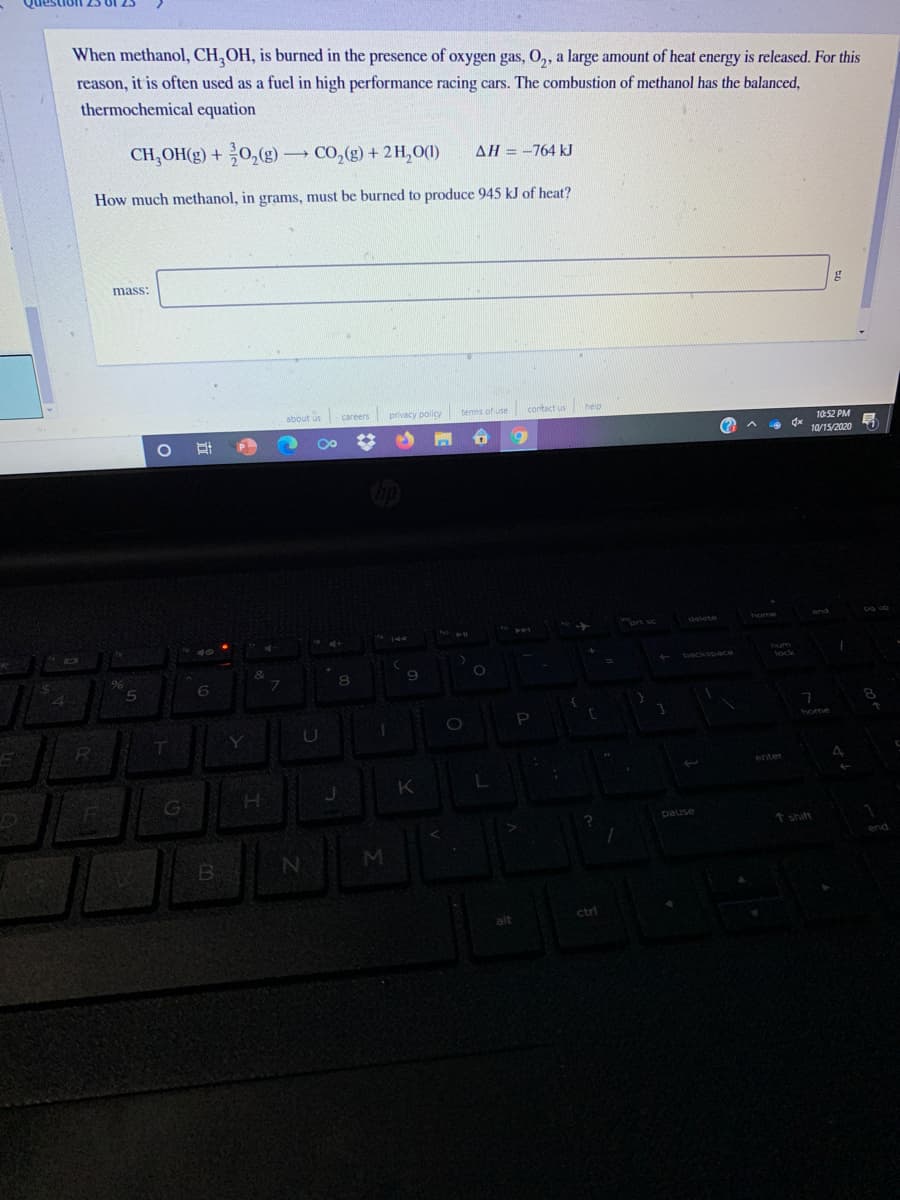
Transcribed Image Text:When methanol, CH,OH, is burned in the presence of oxygen gas, O,, a large amount of heat energy is released. For this
reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced,
thermochemical equation
CH,OH(g) + 0,(g)
- CO, (g) + 2H,O(1)
AH = -764 kJ
How much methanol, in grams, must be burned to produce 945 kJ of heat?
mass:
g
about us
privacy policy tems of use
contact us he
10:52 PM
(?
10/15/2020
ort sc
8.
6.
G
pause
t shift
ctrl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning