Which liquid is the most volatile? most volatile: choose one Which is the least volatile? least volatile: choose one isopropyl acetate: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. ethanol: tetrahydrofuran: more Suppose a beaker of ethanol is put inside a sealed tank containing ethanol gas at 69. degree C and 521. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? less the same
Which liquid is the most volatile? most volatile: choose one Which is the least volatile? least volatile: choose one isopropyl acetate: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. ethanol: tetrahydrofuran: more Suppose a beaker of ethanol is put inside a sealed tank containing ethanol gas at 69. degree C and 521. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? less the same
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 25A
Related questions
Question
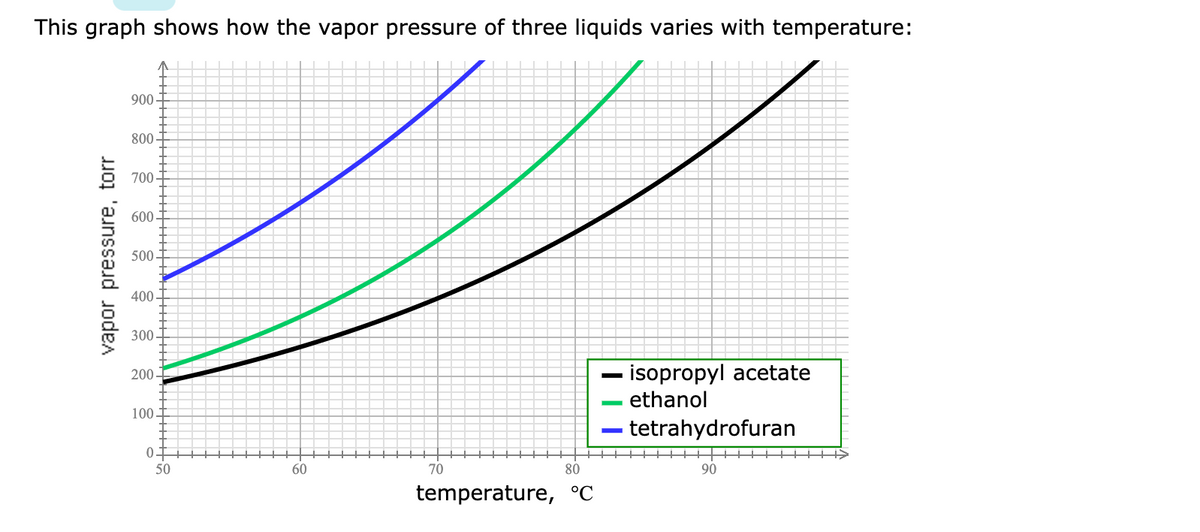
Transcribed Image Text:This graph shows how the vapor pressure of three liquids varies with temperature:
900-
800
700
600-
500
400
300
- isopropyl acetate
ethanol
200
100
tetrahydrofuran
50
60
70
80
90
temperature, °C
vapor pressure, torr
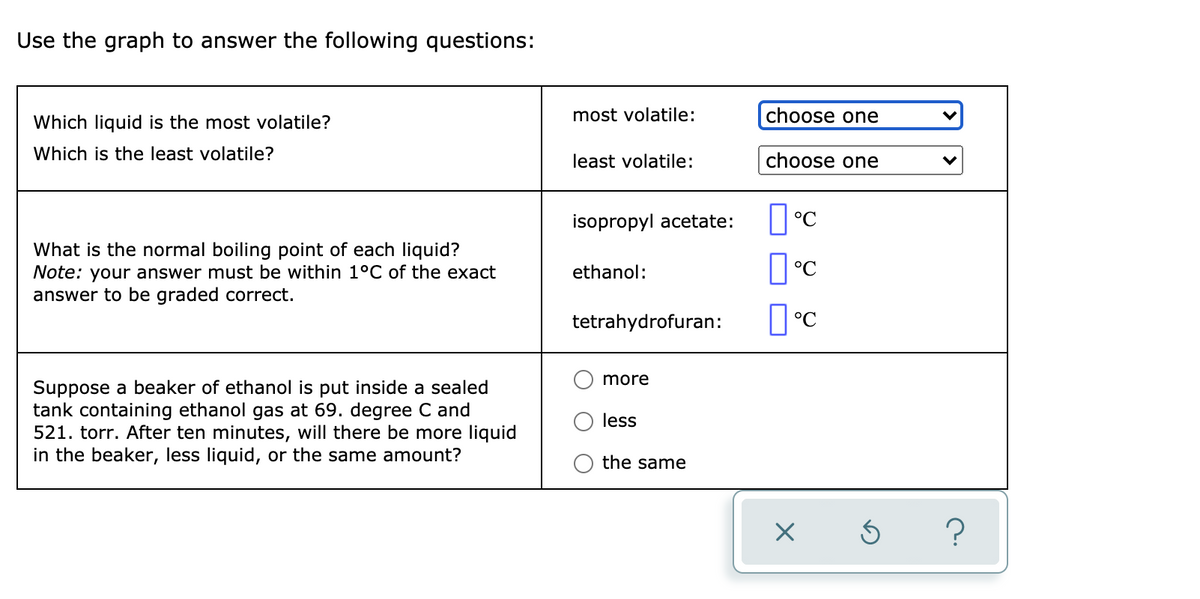
Transcribed Image Text:Use the graph to answer the following questions:
Which liquid is the most volatile?
most volatile:
choose one
Which is the least volatile?
least volatile:
choose one
isopropyl acetate:
What is the normal boiling point of each liquid?
Note: your answer must be within 1°C of the exact
answer to be graded correct.
ethanol:
tetrahydrofuran:
more
Suppose a beaker of ethanol is put inside a sealed
tank containing ethanol gas at 69. degree C and
521. torr. After ten minutes, will there be more liquid
in the beaker, less liquid, or the same amount?
less
the same
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning