Which of the following aqueous solutions will have the lowest freezing point? 0.10 m sodium carbonate, Na2CO3 a. 0.25 m sodium nitrate, NaNO; 0.20 m ethanol, CH;CH;OH d. 0.15 m calcium chloride, CaCl2 0.30 m potassium chloride, KCI b. c. e. Indicate which of the following reactions results in a positive ASays. a. 2 Na(s) +Cl, (g) = NaCl(s) b. Zn(s)+2 HCI(aq) 5 ZnCl, (aq) +H, (g) 4 Fe(s) + 3 0, (g) 5 2 Fe,O,(s) d. 2 H, (g)+O,(g) 5 2 H,O(g) Ba(NO,),(aq) + Na, SO, (aq) 5 BaSO,(s) + 2 NaNO, (aq) c. e.
Which of the following aqueous solutions will have the lowest freezing point? 0.10 m sodium carbonate, Na2CO3 a. 0.25 m sodium nitrate, NaNO; 0.20 m ethanol, CH;CH;OH d. 0.15 m calcium chloride, CaCl2 0.30 m potassium chloride, KCI b. c. e. Indicate which of the following reactions results in a positive ASays. a. 2 Na(s) +Cl, (g) = NaCl(s) b. Zn(s)+2 HCI(aq) 5 ZnCl, (aq) +H, (g) 4 Fe(s) + 3 0, (g) 5 2 Fe,O,(s) d. 2 H, (g)+O,(g) 5 2 H,O(g) Ba(NO,),(aq) + Na, SO, (aq) 5 BaSO,(s) + 2 NaNO, (aq) c. e.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 40E
Related questions
Question
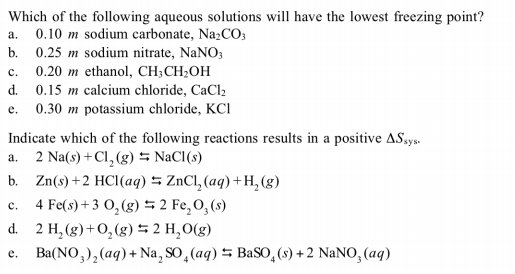
Transcribed Image Text:Which of the following aqueous solutions will have the lowest freezing point?
0.10 m sodium carbonate, Na2CO3
a.
0.25 m sodium nitrate, NaNO;
0.20 m ethanol, CH;CH;OH
d. 0.15 m calcium chloride, CaCl2
0.30 m potassium chloride, KCI
b.
c.
e.
Indicate which of the following reactions results in a positive ASays.
a. 2 Na(s) +Cl, (g) = NaCl(s)
b. Zn(s)+2 HCI(aq) 5 ZnCl, (aq) +H, (g)
4 Fe(s) + 3 0, (g) 5 2 Fe,O,(s)
d. 2 H, (g)+O,(g) 5 2 H,O(g)
Ba(NO,),(aq) + Na, SO, (aq) 5 BaSO,(s) + 2 NaNO, (aq)
c.
e.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning