Which of the following explanations BEST describes how an atom emits color? O A. Protons jump to higher energy levels and specific wavelengths are emitted as the protons return to the ground state. O B. Protons jump to higher energy levels and specific wavelengths are emitted as the protons move to the excited state. O C. Electrons jump to higher energy levels and specific wavelengths are emitted as the electrons return to the ground state. O D. Electrons jump to higher energy levels and specific wavelengths are emitted as the electrons move to the excited state.
Which of the following explanations BEST describes how an atom emits color? O A. Protons jump to higher energy levels and specific wavelengths are emitted as the protons return to the ground state. O B. Protons jump to higher energy levels and specific wavelengths are emitted as the protons move to the excited state. O C. Electrons jump to higher energy levels and specific wavelengths are emitted as the electrons return to the ground state. O D. Electrons jump to higher energy levels and specific wavelengths are emitted as the electrons move to the excited state.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter7: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 67E: Consider only the transitions involving the first four energy levels for a hydrogen atom: a. How...
Related questions
Question
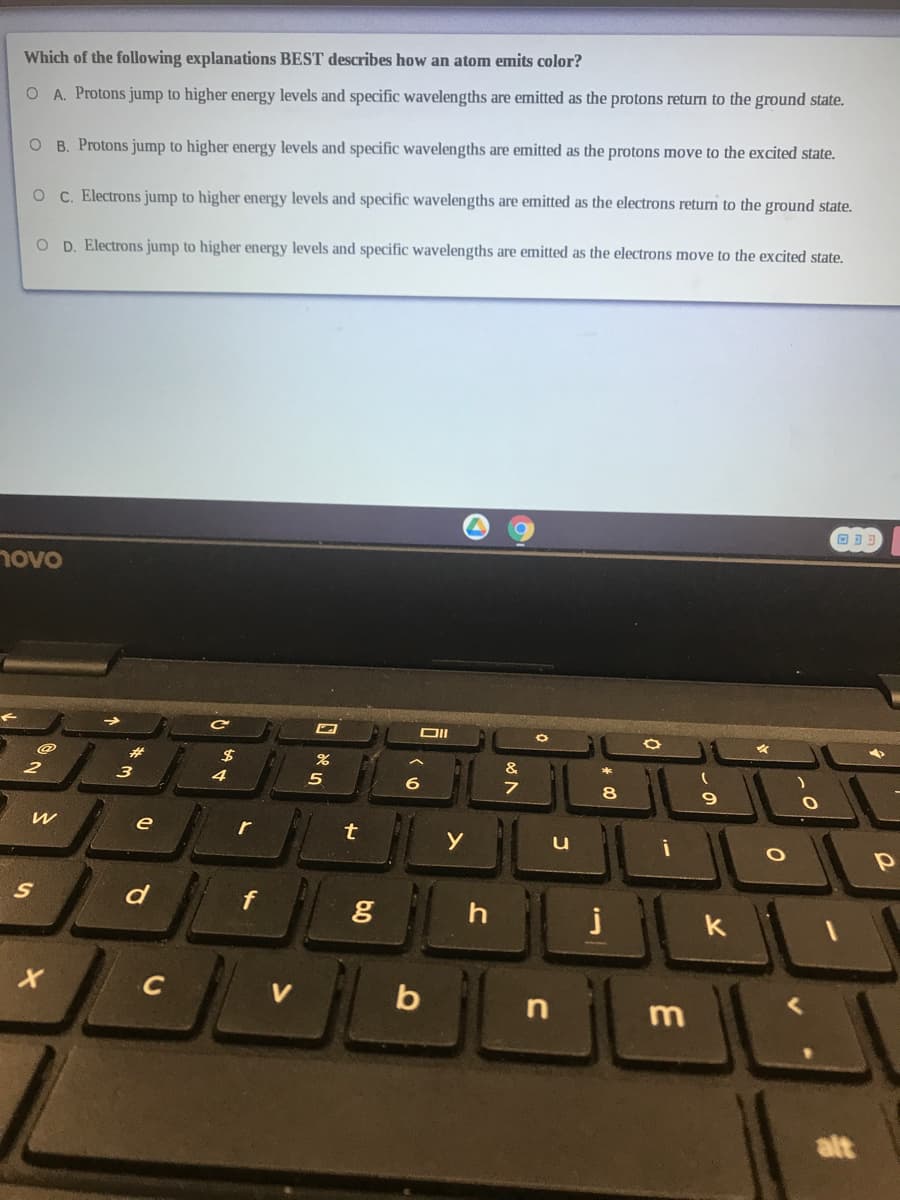
Transcribed Image Text:Which of the following explanations BEST describes how an atom emits color?
O A. Protons jump to higher energy levels and specific wavelengths are emitted as the protons return to the ground state.
O B. Protons jump to higher energy levels and specific wavelengths are emitted as the protons move to the excited state.
O C. Electrons jump to higher energy levels and specific wavelengths are emitted as the electrons return to the ground state.
O D. Electrons jump to higher energy levels and specific wavelengths are emitted as the electrons move to the excited state.
novo
DII
%23
&
3
e
r
t
u
i
d
f
j
k
C
b
n
m
alt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

