Which of the following gas samples would have the largest volume, if all samples are at the same temperature and pressure? O 7x1023 molecules of N2 O 0.202 moles CO2 40.4 grams of Ne O they would all have the same volume FLV P Type here to search home ins prt sc delete (12 f10 fg 18 144 f6 f5 f4 SC backspace 8. 4 Q %24 3. %23 2.
Which of the following gas samples would have the largest volume, if all samples are at the same temperature and pressure? O 7x1023 molecules of N2 O 0.202 moles CO2 40.4 grams of Ne O they would all have the same volume FLV P Type here to search home ins prt sc delete (12 f10 fg 18 144 f6 f5 f4 SC backspace 8. 4 Q %24 3. %23 2.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section10.2: Gas Laws: The Experimental Basis
Problem 2RC: 2. The volume of a gas sample is 235 mL at a temperature of 25 ℃. At what temperature would that...
Related questions
Question
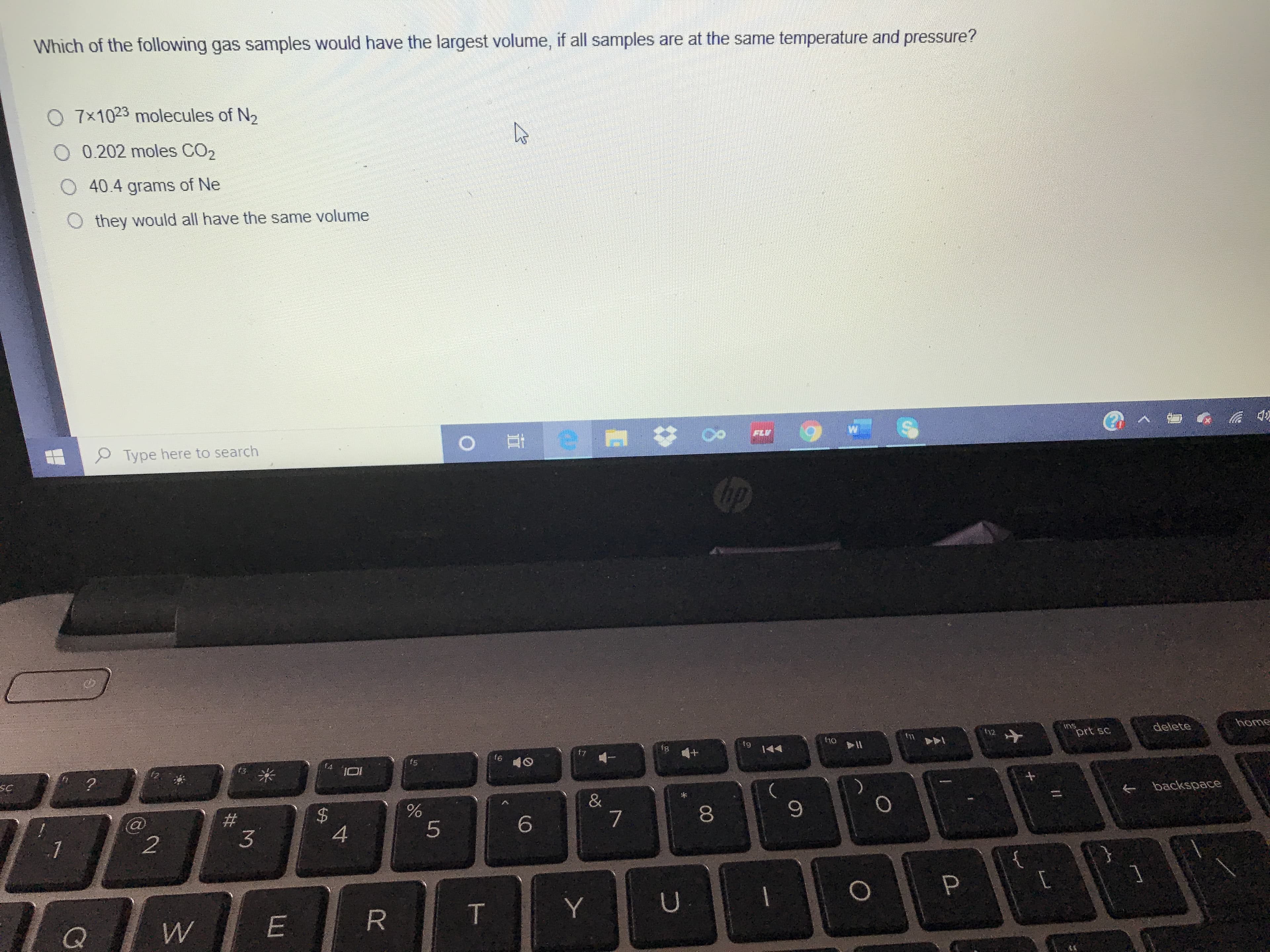
Transcribed Image Text:Which of the following gas samples would have the largest volume, if all samples are at the same temperature and pressure?
O 7x1023 molecules of N2
O 0.202 moles CO2
40.4 grams of Ne
O they would all have the same volume
FLV
P Type here to search
home
ins
prt sc
delete
(12
f10
fg
18
144
f6
f5
f4
SC
backspace
8.
4
Q
%24
3.
%23
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning