Which of the following postulates of the Bohr model led to the quantization of encrgy of the hydrogen atom? A- The electron goes around the nucleus in circular orbits. B- The angular momentum of the electron can only be an integral multiple of h/2a. C- The magnitude of the linear momentum of the electron is quantized. D- Quantization of energy is itself a postulate of the Bohr model.A. Electrons, The Bohr model of atoms A- assumes that the angular momentum of electrons is quantized. B- uses Einstein's photoelectric equation. C- predicts continuous emission spectra for atoms. D- predicts the same emission spectra for all types of atoms. The diagram shows the energy levels for an electron in a certain atom. Which transition shown represents the emission of a photon with the most energy? A- I B- II C- II D- IV
Which of the following postulates of the Bohr model led to the quantization of encrgy of the hydrogen atom? A- The electron goes around the nucleus in circular orbits. B- The angular momentum of the electron can only be an integral multiple of h/2a. C- The magnitude of the linear momentum of the electron is quantized. D- Quantization of energy is itself a postulate of the Bohr model.A. Electrons, The Bohr model of atoms A- assumes that the angular momentum of electrons is quantized. B- uses Einstein's photoelectric equation. C- predicts continuous emission spectra for atoms. D- predicts the same emission spectra for all types of atoms. The diagram shows the energy levels for an electron in a certain atom. Which transition shown represents the emission of a photon with the most energy? A- I B- II C- II D- IV
Related questions
Question
Choose the correct answer
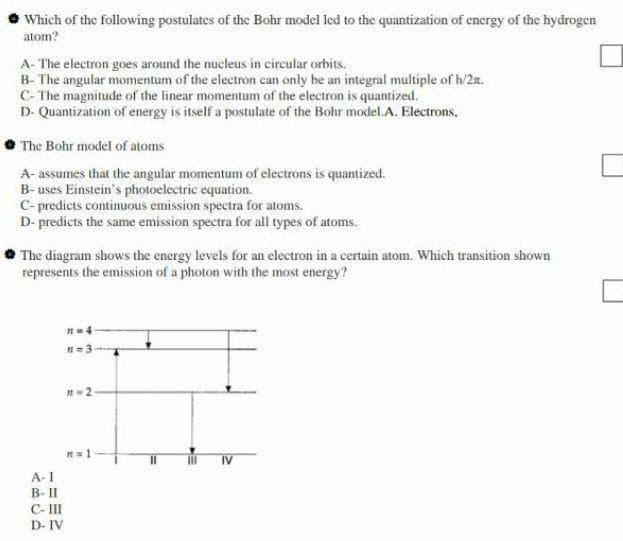
Transcribed Image Text:Which of the following postulates of the Bohr model led to the quantization of encrgy of the hydrogen
atom?
A- The electron goes around the nucleus in circular orbits.
B- The angular momentum of the electron can only be an integral multiple of h/2a.
C- The magnitude of the linear momentum of the electron is quantized.
D- Quantization of energy is itself a postulate of the Bohr model.A. Electrons,
The Bohr model of atoms
A- assumes that the angular momentum of electrons is quantized.
B- uses Einstein's photoelectric equation.
C- predicts continuous emission spectra for atoms.
D- predicts the same emission spectra for all types of atoms.
The diagram shows the energy levels for an electron in a certain atom. Which transition shown
represents the emission of a photon with the most energy?
A- I
B- II
C- II
D- IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps
