Which one of these statements is NOT TRUE? The steric number determines the canonical shape of the molecule, which is called the electron-domain geometry. The energy of a molecule is lowered if the repulsions between bonding & nonbonding electron pairs are minimized for all atoms in the molecule. Nonbonding electron pairs on an atom repel each other electrostatically, but bonding pairs do not. In VSEPR theory, all of the electron pairs centered on an atom are placed as far as possible from each other in three dimensions. O The shape of the molecules is determined by the geometry of the electron domains.
Which one of these statements is NOT TRUE? The steric number determines the canonical shape of the molecule, which is called the electron-domain geometry. The energy of a molecule is lowered if the repulsions between bonding & nonbonding electron pairs are minimized for all atoms in the molecule. Nonbonding electron pairs on an atom repel each other electrostatically, but bonding pairs do not. In VSEPR theory, all of the electron pairs centered on an atom are placed as far as possible from each other in three dimensions. O The shape of the molecules is determined by the geometry of the electron domains.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter1: Bond Angles And Shape
Section: Chapter Questions
Problem 12CTQ: Consider the following flat drawing of methane (CH4) . a. What is HCH bond angle implied by this...
Related questions
Question
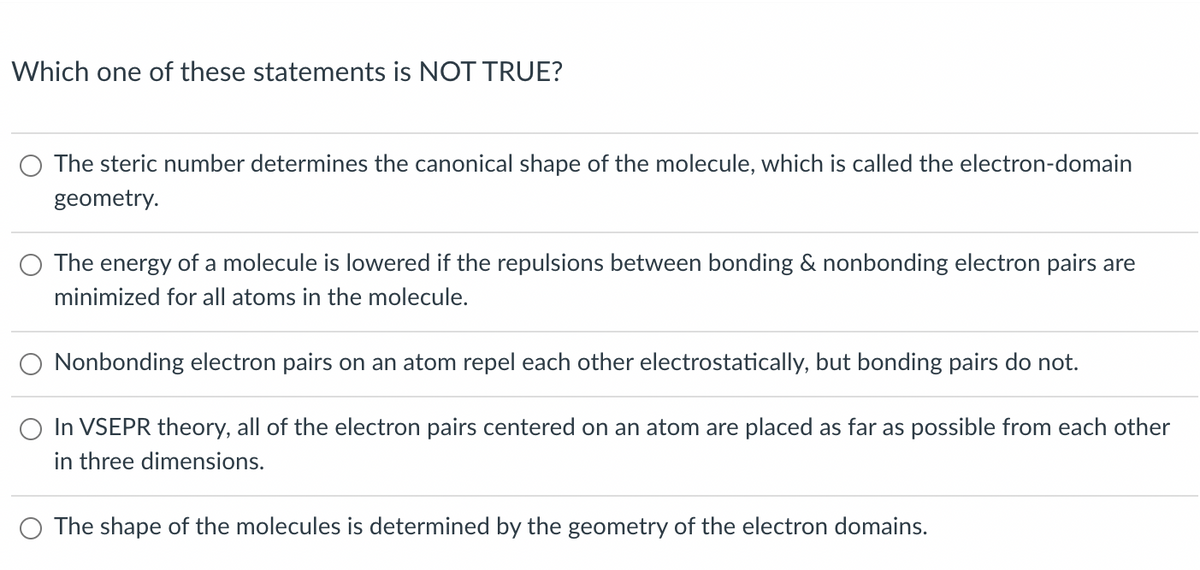
Transcribed Image Text:Which one of these statements is NOT TRUE?
O The steric number determines the canonical shape of the molecule, which is called the electron-domain
geometry.
The energy of a molecule is lowered if the repulsions between bonding & nonbonding electron pairs are
minimized for all atoms in the molecule.
O Nonbonding electron pairs on an atom repel each other electrostatically, but bonding pairs do not.
O In VSEPR theory, all of the electron pairs centered on an atom are placed as far as possible from each other
in three dimensions.
O The shape of the molecules is determined by the geometry of the electron domains.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
