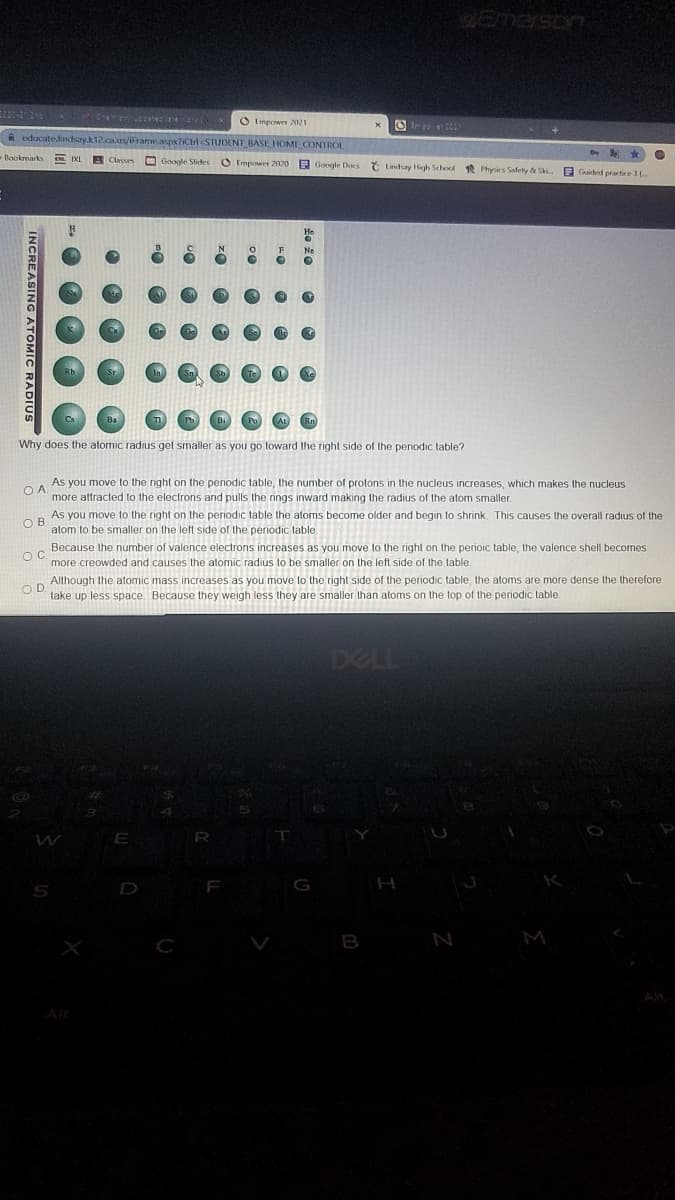
Why does the atomic radius get smaller as you go toward the right side of the penodic table? As you move to the right on the penodic table, the number of protons in the nucleus increases, which makes the nucleus more attracted to the electrons and pulls the nings inward making the radius of the atom smaller. O A As you move to the right on the periodic table the atoms become older and begin to shrink This causes the overall radius of the atom to be smaller on the left side of the periodic table O B Because the number of valence electrons increases as you move to the right on the perioic table, the valence shell becomes more creowded and causes the atomic radius to be smaller on the left side of the table. OD. Although the atomic mass increases as you move to the right side of the periodic table, the atoms are more dense the therefore take up less space. Because they weigh less they are smaller than aloms on the top of the periodic table.
Why does the atomic radius get smaller as you go toward the right side of the penodic table? As you move to the right on the penodic table, the number of protons in the nucleus increases, which makes the nucleus more attracted to the electrons and pulls the nings inward making the radius of the atom smaller. O A As you move to the right on the periodic table the atoms become older and begin to shrink This causes the overall radius of the atom to be smaller on the left side of the periodic table O B Because the number of valence electrons increases as you move to the right on the perioic table, the valence shell becomes more creowded and causes the atomic radius to be smaller on the left side of the table. OD. Although the atomic mass increases as you move to the right side of the periodic table, the atoms are more dense the therefore take up less space. Because they weigh less they are smaller than aloms on the top of the periodic table.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 102AP
Related questions
Question
Transcribed Image Text:Ce CEstelae et
O Empower 2021
O ir
A educate.lindsay.k12.ca.us/iFrame.aspx?iCtrl-STUDENT BASE HOME CONTROL
Bookmarks
IXI
O Impower 2020 E Google Docs č indsay High School R Physics Safety & Sk.
E Gided practice 3L
Why does the atomic radius get smaller as you go toward the right side of the penodic table?
As you move to the right on the penodic table, the number of protons in the nucleus increases, which makes the nucleus
O A
more attracted to the electrons and pulls the rings inward making the radius of the atom smaller.
As you move to the right on the periodic table the atoms become older and begin to shrink. This causes the overall radius of the
atom to be smaller on the left side of the periodic table.
Because the number
valence electrons increases as you move to the right on the perioic table, the valence shell becomes
more creowded and causes the atomic radius to be smaller on the left side of the table.
Although the atomic mass increases as you move to the right side of the periodic table, the atoms are more dense the therefore
O D
take up less space. Because they weigh less they are smaller than aloms on the top of the periodic table.
DELL
E
R
D
F
B
INCREASING ATOMIC RADIUS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
