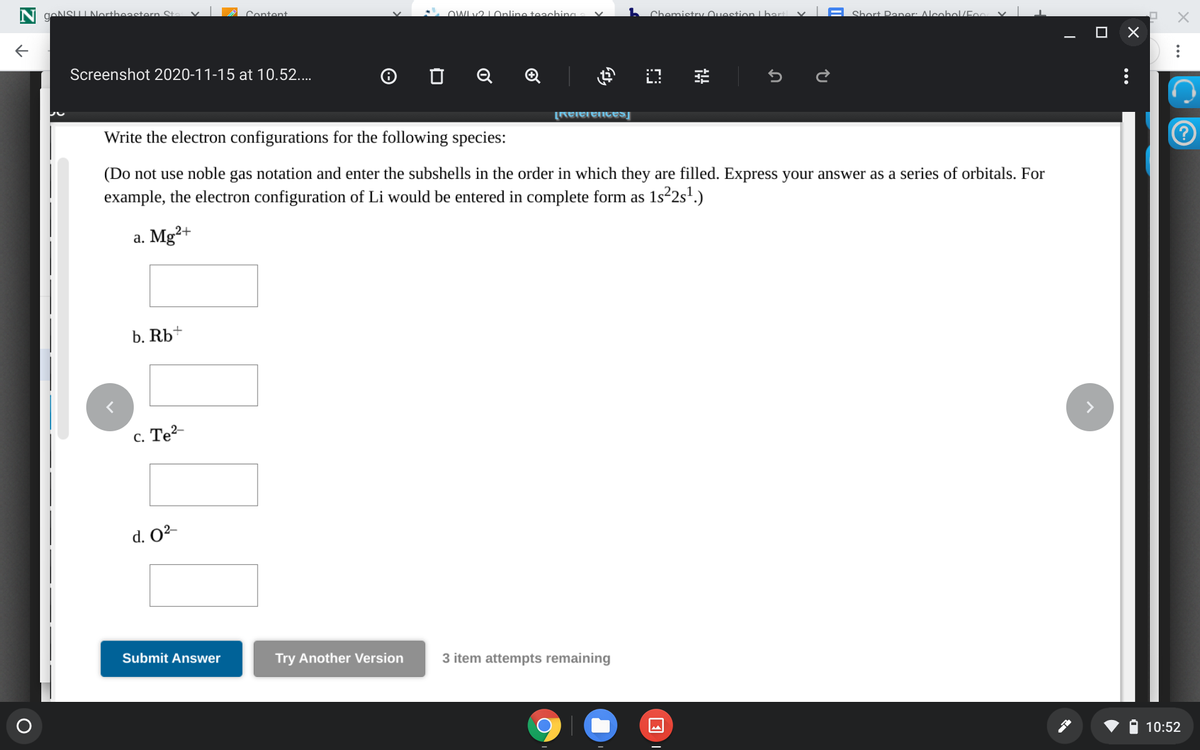
Write the electron configurations for the following species: (Do not use noble gas notation and enter the subshells in the order in which they are filled. Express your answer as a series of orbitals. For example, the electron configuration of Li would be entered in complete form as 1s²2s'.) a. Mg²+ b. Rb* с. Те2- > d. O²-
Write the electron configurations for the following species: (Do not use noble gas notation and enter the subshells in the order in which they are filled. Express your answer as a series of orbitals. For example, the electron configuration of Li would be entered in complete form as 1s²2s'.) a. Mg²+ b. Rb* с. Те2- > d. O²-
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 139QRT
Related questions
Question
Transcribed Image Text:geNSUINortheastern St
Content
OWLv2 LOnline teaching.
Chomistry uestion Lbart|
vLAShort Panor: Alcohol/Foo
O X
Screenshot 2020-11-15 at 10.52...
O O Q Q
Write the electron configurations for the following species:
(Do not use noble gas notation and enter the subshells in the order in which they are filled. Express your answer as a series of orbitals. For
example, the electron configuration of Li would be entered in complete form as 1s²2s².)
a. Mg²+
b. Rb+
с. Те2-
d. O²-
Submit Answer
Try Another Version
3 item attempts remaining
10:52
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning