Write the expressions for K, for the following reactions. In each case indicate whether the reaction is homoge- neous or heterogeneous. (a) 203(g) = 302(8) (b) Ti(s) + 2 Cl2(8) = TIC14(I) (c) 2 C,H,(8) + 2H,0(g) = 2 C,H¿(8) + O2(8)| (d) C(s) + 2 H2(8) = CH4(8) (e) 4 HCI(aq) + Oz(8) = 2 H20(1) + 2 Cl2(8) (f) 2 C3H18(1) + 25 O2(g) = 16 CO2(8) + 18 H20(g) (g) 2 C3H18(1) + 25 O2(8) 16 CO2(8) + 18 H20(I)
Write the expressions for K, for the following reactions. In each case indicate whether the reaction is homoge- neous or heterogeneous. (a) 203(g) = 302(8) (b) Ti(s) + 2 Cl2(8) = TIC14(I) (c) 2 C,H,(8) + 2H,0(g) = 2 C,H¿(8) + O2(8)| (d) C(s) + 2 H2(8) = CH4(8) (e) 4 HCI(aq) + Oz(8) = 2 H20(1) + 2 Cl2(8) (f) 2 C3H18(1) + 25 O2(g) = 16 CO2(8) + 18 H20(g) (g) 2 C3H18(1) + 25 O2(8) 16 CO2(8) + 18 H20(I)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 28E: Write the expression of the reaction quotient for the ionization of HOCN in water.
Related questions
Question
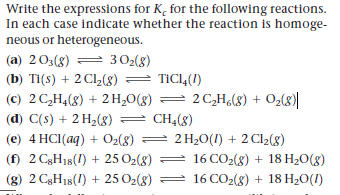
Transcribed Image Text:Write the expressions for K, for the following reactions.
In each case indicate whether the reaction is homoge-
neous or heterogeneous.
(a) 203(g)
= 302(8)
(b) Ti(s) + 2 Cl2(8) = TIC14(I)
(c) 2 C,H,(8) + 2H,0(g) = 2 C,H¿(8) + O2(8)|
(d) C(s) + 2 H2(8) = CH4(8)
(e) 4 HCI(aq) + Oz(8) = 2 H20(1) + 2 Cl2(8)
(f) 2 C3H18(1) + 25 O2(g) =
16 CO2(8) + 18 H20(g)
(g) 2 C3H18(1) + 25 O2(8)
16 CO2(8) + 18 H20(I)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning