Write The Molecular Fotmula For The Following 1-Copper Sulfate 2-Phospours Pentabromide 3-Calicum Chloride dihydrate * 4-Sodium Oxide إجابتك A- Balance The Equation NO2(g) + H2O(1) -- HNO3(1) + NO(g) B- What is formula mass of K2Cr207 (O=16g/mol , Cr=52g/mol and K=39g/mol) C- Calculate the empirical ----- formula of a C6H12O6 that following present composition by mass: C=23.75%, H=37.5% and O=40% percent. (C=12g/mol, * H=1g/mol and O=16g/mol) إجابتك What is mean the Base according to -1 Arrhenius and the Acid acoording to Bronsted ? 2- What the solvent of 100ml N2 and 25ml 02 ? Why? 3-What is Oxidation Number of ions in these compouds a- * KMNO4 b-MgH2 c-H2O2 d-N2 e-CIO3 -1
Write The Molecular Fotmula For The Following 1-Copper Sulfate 2-Phospours Pentabromide 3-Calicum Chloride dihydrate * 4-Sodium Oxide إجابتك A- Balance The Equation NO2(g) + H2O(1) -- HNO3(1) + NO(g) B- What is formula mass of K2Cr207 (O=16g/mol , Cr=52g/mol and K=39g/mol) C- Calculate the empirical ----- formula of a C6H12O6 that following present composition by mass: C=23.75%, H=37.5% and O=40% percent. (C=12g/mol, * H=1g/mol and O=16g/mol) إجابتك What is mean the Base according to -1 Arrhenius and the Acid acoording to Bronsted ? 2- What the solvent of 100ml N2 and 25ml 02 ? Why? 3-What is Oxidation Number of ions in these compouds a- * KMNO4 b-MgH2 c-H2O2 d-N2 e-CIO3 -1
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
Transcribed Image Text:%TE l. a
9:-1
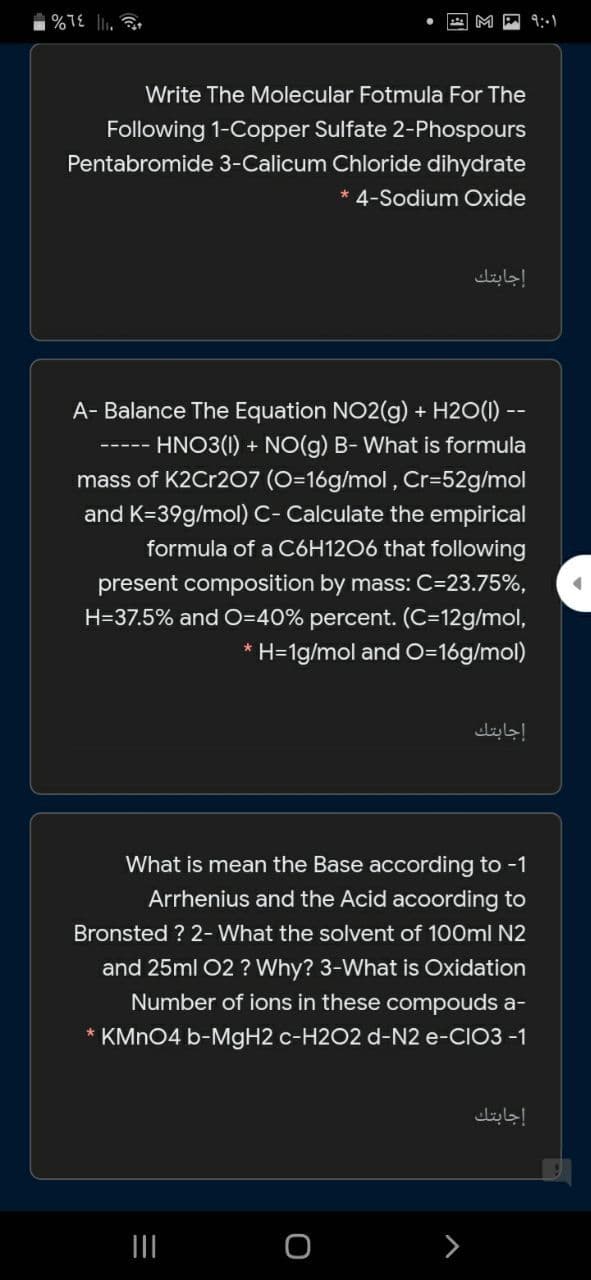
Write The Molecular Fotmula For The
Following 1-Copper Sulfate 2-Phospours
Pentabromide 3-Calicum Chloride dihydrate
*4-Sodium Oxide
إجابتك
A- Balance The Equation NO2(g) + H2O(1) --
HNO3(1) + NO(g) B- What is formula
mass of K2Cr2O7 (O=16g/mol , Cr=52g/mol
and K=39g/mol) C- Calculate the empirical
formula of a C6H12O6 that following
present composition by mass: C=23.75%,
H=37.5% and O=40% percent. (C=12g/mol,
* H=1g/mol and O=16g/mol)
إجابتك
What is mean the Base according to -1
Arrhenius and the Acid acoording to
Bronsted ? 2- What the solvent of 100ml N2
and 25ml 02 ? Why? 3-What is Oxidation
Number of ions in these compouds a-
* KMN04 b-MGH2 c-H2O2 d-N2 e-CIO3 -1
إجابتك
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT