www-awn.aleks.com Significant Figures Counter Sign out Sign out My Courses-Blackboard... https://learn-us-east-1-pr... ALEKS Sabina Lamgadh... https://gtu.ge/Agro-Lib/M O CHEMICAL REACTIONS Sabina Solving for a reactant using a chemical equation Ammonium phosphate ((NH) PO,) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia (NH3). What mass of ammonium phosphate is produced by the reaction of 4.98 g of phosphoric acid? Be sure your answer has the correct number of significant digits. do x10 Ar Check Privacy Terms of Use Explanation © 2020 McGraw Hill Education, All Rights Reserved. P. MacBook Air F10 F9 F8 F7 F6 F5 20 F3 F4 F1 & * cQ %24 II
www-awn.aleks.com Significant Figures Counter Sign out Sign out My Courses-Blackboard... https://learn-us-east-1-pr... ALEKS Sabina Lamgadh... https://gtu.ge/Agro-Lib/M O CHEMICAL REACTIONS Sabina Solving for a reactant using a chemical equation Ammonium phosphate ((NH) PO,) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia (NH3). What mass of ammonium phosphate is produced by the reaction of 4.98 g of phosphoric acid? Be sure your answer has the correct number of significant digits. do x10 Ar Check Privacy Terms of Use Explanation © 2020 McGraw Hill Education, All Rights Reserved. P. MacBook Air F10 F9 F8 F7 F6 F5 20 F3 F4 F1 & * cQ %24 II
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.14QAP
Related questions
Question
Transcribed Image Text:www-awn.aleks.com
Significant Figures Counter
Sign out
Sign out
My Courses-Blackboard...
https://learn-us-east-1-pr...
ALEKS Sabina Lamgadh...
https://gtu.ge/Agro-Lib/M
O CHEMICAL REACTIONS
Sabina
Solving for a reactant using a chemical equation
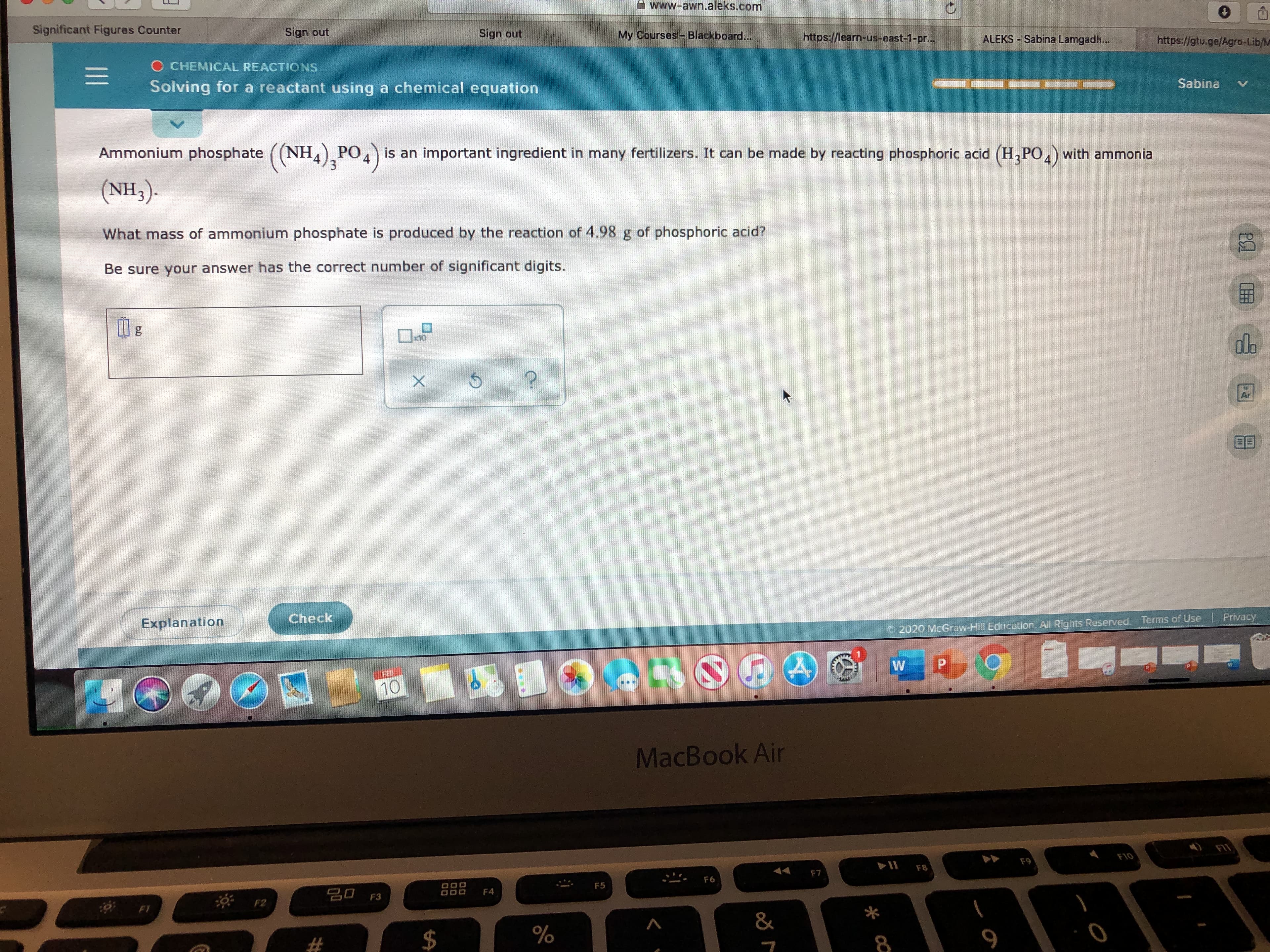
Ammonium phosphate ((NH) PO,) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia
(NH3).
What mass of ammonium phosphate is produced by the reaction of 4.98 g of phosphoric acid?
Be sure your answer has the correct number of significant digits.
do
x10
Ar
Check
Privacy
Terms of Use
Explanation
© 2020 McGraw Hill Education, All Rights Reserved.
P.
MacBook Air
F10
F9
F8
F7
F6
F5
20 F3
F4
F1
&
* cQ
%24
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

