X 101 Chem 101 (71) Tyler DeWitt - YouTube X app.101edu.co Submit Question 25 of 47 How many moles of MnOs are produced when 4.30 kg of oxygen gas completely reacts according to the balanced chemical reaction: MnOs(s) O2(g) 2 Mn(s) 33 2 2 mol MnO3 kgo 2 mol MnOs 4.30 X = kg O. STARTING AMOUNT 2 mol MnOs = 2 mol MnO 4.30 kg O2 x 3 kg O2 RESET ADD FACTOR DELETE ANSWER -) X X 6.022 x 1023 0.001 4.30 9.22 x 103 32.00 3 0.0896 54.94 1000 2 89.6 102.94 g O2 mol Mn mol O2 mol MnOs kg O2 g MnOs g Mn 9:06 PM е OTYPE here to search 10/31/2019
X 101 Chem 101 (71) Tyler DeWitt - YouTube X app.101edu.co Submit Question 25 of 47 How many moles of MnOs are produced when 4.30 kg of oxygen gas completely reacts according to the balanced chemical reaction: MnOs(s) O2(g) 2 Mn(s) 33 2 2 mol MnO3 kgo 2 mol MnOs 4.30 X = kg O. STARTING AMOUNT 2 mol MnOs = 2 mol MnO 4.30 kg O2 x 3 kg O2 RESET ADD FACTOR DELETE ANSWER -) X X 6.022 x 1023 0.001 4.30 9.22 x 103 32.00 3 0.0896 54.94 1000 2 89.6 102.94 g O2 mol Mn mol O2 mol MnOs kg O2 g MnOs g Mn 9:06 PM е OTYPE here to search 10/31/2019
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter20: Organic Chemistry
Section: Chapter Questions
Problem 37E: MTBE, Methyl tert -butyl ether, CH3OC(CH3)3, is used as an oxygen source in oxygenated gasolines....
Related questions
Question
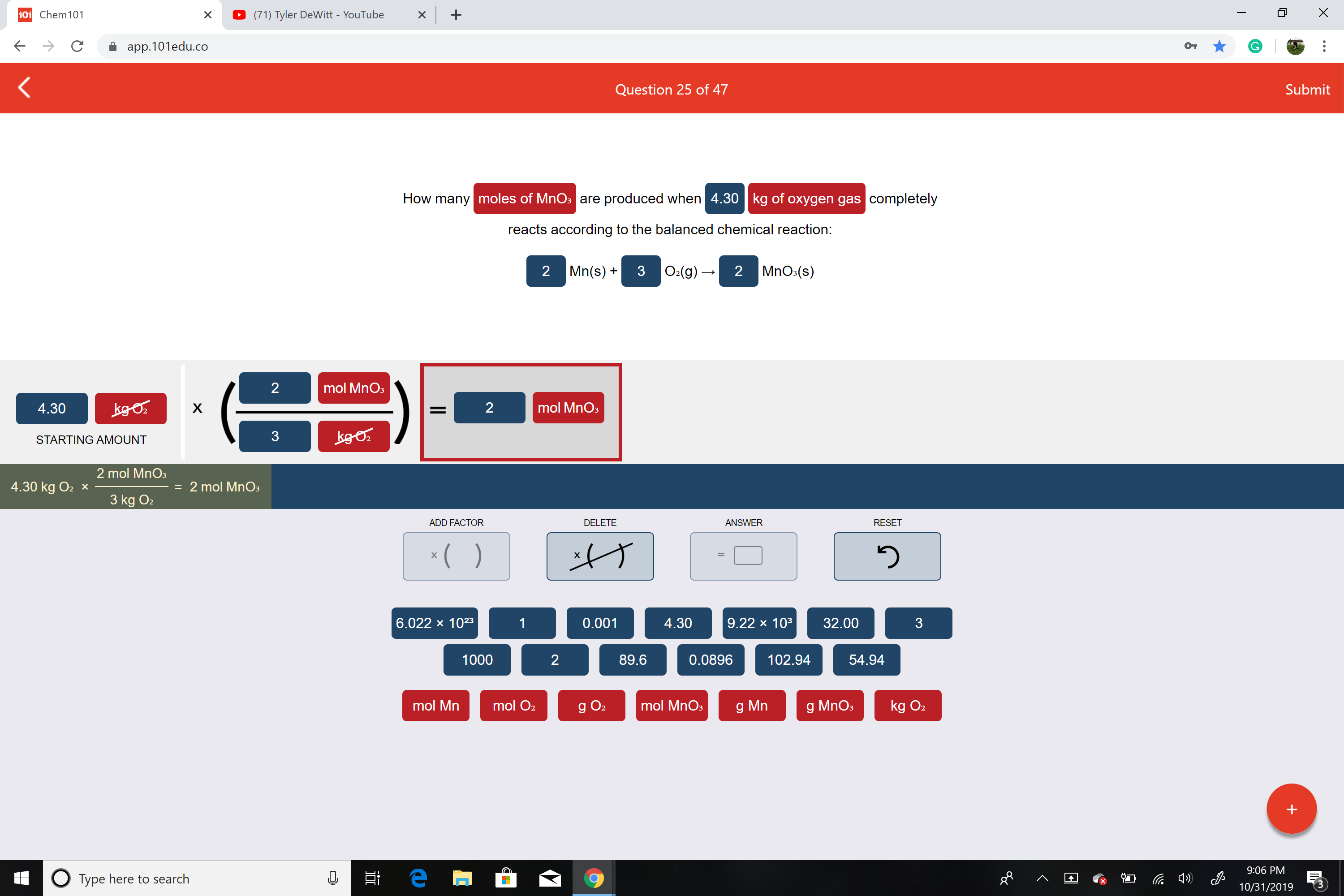
Transcribed Image Text:X
101 Chem 101
(71) Tyler DeWitt - YouTube
X
app.101edu.co
Submit
Question 25 of 47
How many moles of MnOs are produced when 4.30 kg of oxygen gas completely
reacts according to the balanced chemical reaction:
MnOs(s)
O2(g)
2
Mn(s)
33
2
2
mol MnO3
kgo
2
mol MnOs
4.30
X
=
kg O.
STARTING AMOUNT
2 mol MnOs
= 2 mol MnO
4.30 kg O2 x
3 kg O2
RESET
ADD FACTOR
DELETE
ANSWER
-)
X
X
6.022 x 1023
0.001
4.30
9.22 x 103
32.00
3
0.0896
54.94
1000
2
89.6
102.94
g O2
mol Mn
mol O2
mol MnOs
kg O2
g MnOs
g Mn
9:06 PM
е
OTYPE here to search
10/31/2019
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning