X X Experimer 0 Mail Dirk X Experiment 10 B Homepag G sodium + + Yuzu: Safe X https://www.the collegegateway.com/ChemWeb/site/StudentFormView.php?sectionld=327&L... : https://www.thecollegegateway.com/ChemWeb/site/images/assignments/2/Experiment9%20.. C Table 10.4 cont. B. Freezing-Point Depression -30 Table 10.4 (data) Sodium Chloride Solute Ethylene Glycol Solute Mass of beaker ice water 111.438 112.337 g Mass of empty beaker 58.650 g Freezing point of pure wateroC Mass of sample + container 7.686 g Mass of empty container 2.676 g 53.263 c - 20 0 C 33.185 g 28.524 g Freezing point of solution6 C -3 C 10 Table 10.5 (report) Sodium Chloride Solute Ethylene Glycol Solute Total water mass Mass of solute Moles of solute ?t Water mass in kg 10 Solution molality K C. Boiling-Point Elevation -20 Table 10.6 (data) Boiling point of pure water Freeing point of solution, ethylene glycol solute Sucrose Solute Ethylene Glycol Solute Volume of water used Note: Remember, temperatures below zero should be read -1,-2, -3 as you move down the thermometer scale, where each mark below zero corresponds to -1°C Mass of solute container Mass of empty container Boiling point of solution All photos are Copyrighted by Maren Hansen. 10:14 AM OTYPE here to search 4/4/2019
X X Experimer 0 Mail Dirk X Experiment 10 B Homepag G sodium + + Yuzu: Safe X https://www.the collegegateway.com/ChemWeb/site/StudentFormView.php?sectionld=327&L... : https://www.thecollegegateway.com/ChemWeb/site/images/assignments/2/Experiment9%20.. C Table 10.4 cont. B. Freezing-Point Depression -30 Table 10.4 (data) Sodium Chloride Solute Ethylene Glycol Solute Mass of beaker ice water 111.438 112.337 g Mass of empty beaker 58.650 g Freezing point of pure wateroC Mass of sample + container 7.686 g Mass of empty container 2.676 g 53.263 c - 20 0 C 33.185 g 28.524 g Freezing point of solution6 C -3 C 10 Table 10.5 (report) Sodium Chloride Solute Ethylene Glycol Solute Total water mass Mass of solute Moles of solute ?t Water mass in kg 10 Solution molality K C. Boiling-Point Elevation -20 Table 10.6 (data) Boiling point of pure water Freeing point of solution, ethylene glycol solute Sucrose Solute Ethylene Glycol Solute Volume of water used Note: Remember, temperatures below zero should be read -1,-2, -3 as you move down the thermometer scale, where each mark below zero corresponds to -1°C Mass of solute container Mass of empty container Boiling point of solution All photos are Copyrighted by Maren Hansen. 10:14 AM OTYPE here to search 4/4/2019
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
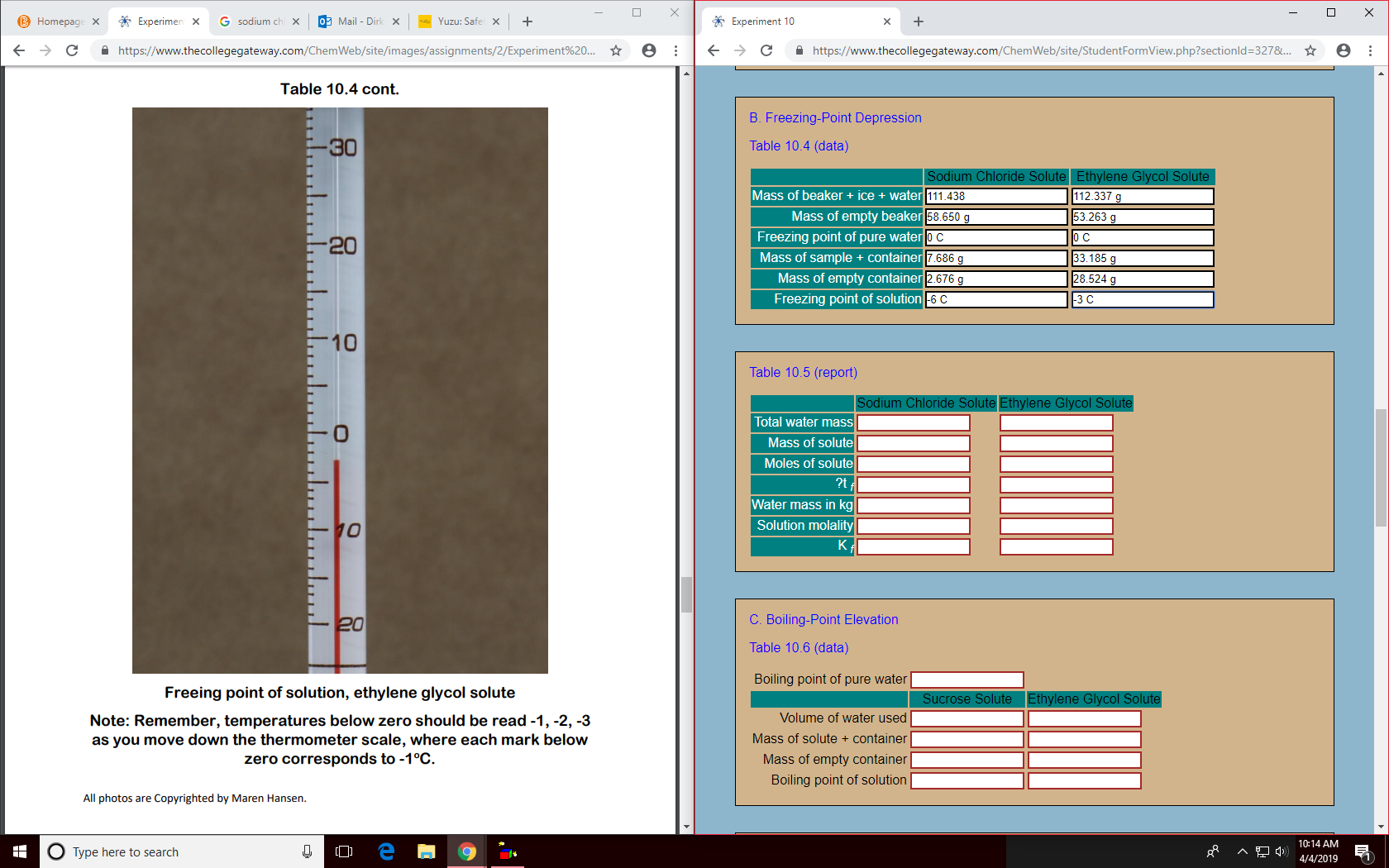
Transcribed Image Text:X
X
Experimer
0 Mail Dirk X
Experiment 10
B Homepag
G sodium
+
+
Yuzu: Safe X
https://www.the collegegateway.com/ChemWeb/site/StudentFormView.php?sectionld=327&L...
:
https://www.thecollegegateway.com/ChemWeb/site/images/assignments/2/Experiment9%20..
C
Table 10.4 cont.
B. Freezing-Point Depression
-30
Table 10.4 (data)
Sodium Chloride Solute Ethylene Glycol Solute
Mass of beaker ice
water 111.438
112.337 g
Mass of empty beaker 58.650 g
Freezing point of pure wateroC
Mass of sample + container 7.686 g
Mass of empty container 2.676 g
53.263 c
- 20
0 C
33.185 g
28.524 g
Freezing point of solution6 C
-3 C
10
Table 10.5 (report)
Sodium Chloride Solute Ethylene Glycol Solute
Total water mass
Mass of solute
Moles of solute
?t
Water mass in kg
10
Solution molality
K
C. Boiling-Point Elevation
-20
Table 10.6 (data)
Boiling point of pure water
Freeing point of solution, ethylene glycol solute
Sucrose Solute
Ethylene Glycol Solute
Volume of water used
Note: Remember, temperatures below zero should be read -1,-2, -3
as you move down the thermometer scale, where each mark below
zero corresponds to -1°C
Mass of solute container
Mass of empty container
Boiling point of solution
All photos are Copyrighted by Maren Hansen.
10:14 AM
OTYPE here to search
4/4/2019
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 10 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY