You are given the phase diagram shown and asked to determine several properties of the substance. First, find the normal melting point and boiling point. 1.00-
You are given the phase diagram shown and asked to determine several properties of the substance. First, find the normal melting point and boiling point. 1.00-
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 15QAP
Related questions
Question
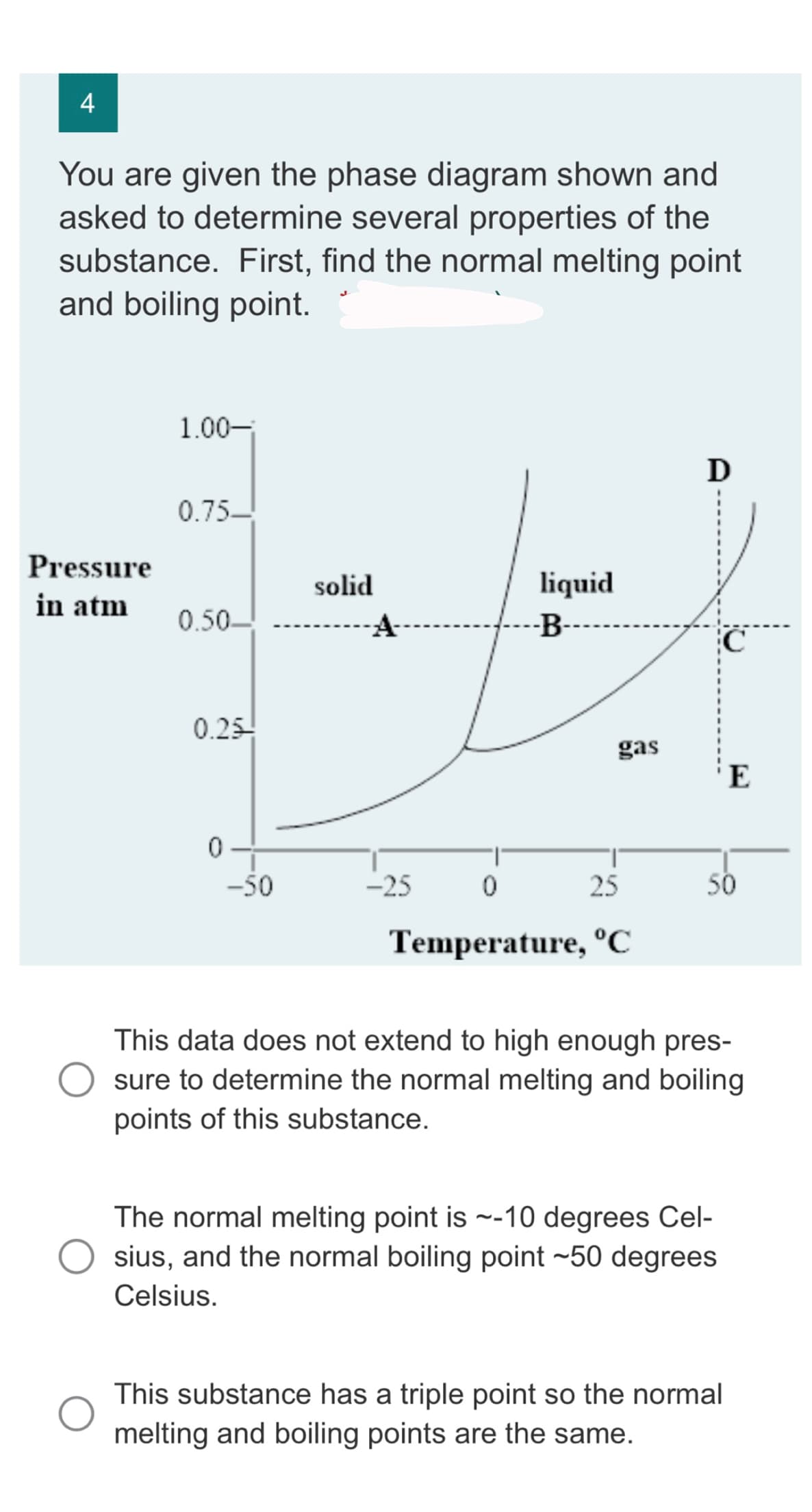
Transcribed Image Text:4
You are given the phase diagram shown and
asked to determine several properties of the
substance. First, find the normal melting point
and boiling point.
Pressure
in atm
1.00-
0.75-
0.50-
0.25-
0
-50
solid
-25
liquid
-B-
gas
0
25
Temperature, °C
D
50
E
This data does not extend to high enough pres-
sure to determine the normal melting and boiling
points of this substance.
The normal melting point is ~-10 degrees Cel-
sius, and the normal boiling point -50 degrees
Celsius.
This substance has a triple point so the normal
melting and boiling points are the same.
Expert Solution
Step 1
Boiling point- The temperature at which vapour pressure of liquid become equal to atmospheric pressure (1 atm) is called boiling point of liquid.
Similarly for melting point
Melting point- The temperature at which pressure of solid become equal to atmospheric pressure (1 atm) is called melting point of solid.
In order to determine the melting and boiling point from the phase diagram, the temperature where binary phase lines meats at 1 atm pressure called melting and boiling point.
In present graph the phase lines are not extended up to atmospheric pressure (1 atm), hence normal melting and boiling point of substance can not be determined from given phase diagram.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning