Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter21: Heat And The First Law Of Thermodynamics
Section: Chapter Questions
Problem 81PQ
Related questions
Question
100%
You have 45 grams of ice at -15 oC that you want to convert into steam at 115 oC. Find ΣQ.
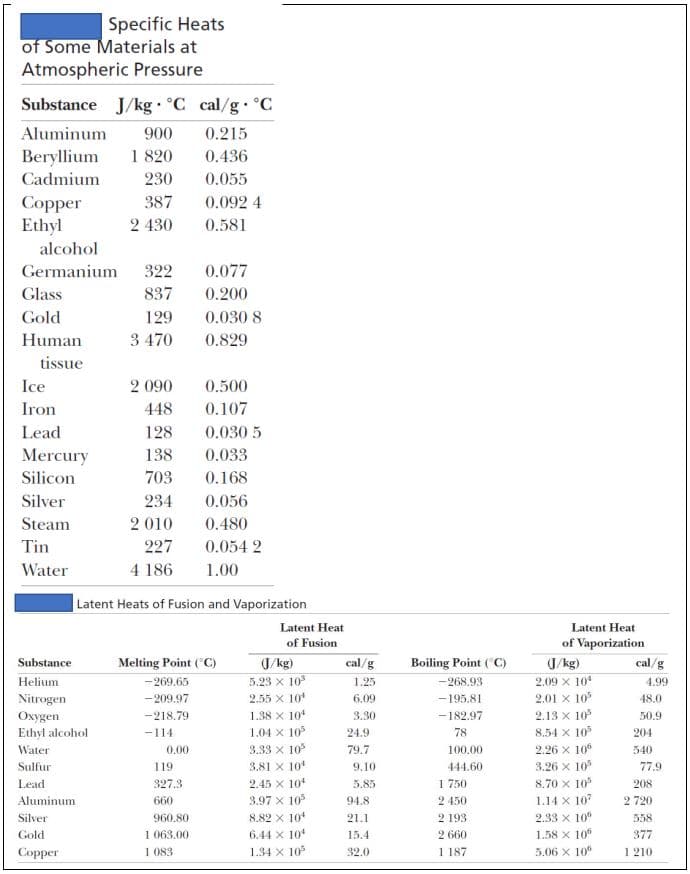
Transcribed Image Text:Specific Heats
of Some Materials at
Atmospheric Pressure
Substance J/kg · °C cal/g °C
Aluminum
900
0.215
Веryllium
1 820
0.436
Cadmium
230
0.055
Copper
Ethyl
387
0.092 4
2 430
0.581
alcohol
Germanium
322
0.077
Glass
837
0.200
Gold
129
0.030 8
Human
3 470
0.829
tissue
Ice
2 090
0.500
Iron
448
0.107
Lead
128
0.030 5
Mercury
138
0.033
Silicon
703
0.168
Silver
234
0.056
Steam
2 010
0.480
Tin
227
0.054 2
Water
4 186
1.00
Latent Heats of Fusion and Vaporization
Latent Heat
of Fusion
Latent Heat
of Vaporization
Substance
Melting Point ("C)
J/kg)
cal/g
Boiling Point (C)
J/kg)
2.09 x 10
2.01 x 10
2.13 x 10
8.54 X 10
cal/g
Helium
-269.65
5.23 x 10
1.25
-268.93
4.99
Nitrogen
Oxygen
Ethyl alcohol
-209.97
2.55 X 10
6.09
-195.81
48.0
-218.79
1.38 x 104
3.30
-182.97
50.9
-114
1.04 x 10
24.9
78
204
Water
0.00
3.33 X 10
79.7
100.00
2.26 x 10
540
3.81 X 10
2.45 X 10
3.26 x 10
8.70 x 10
Sulfur
119
9.10
444.60
77.9
1 750
2 450
Lead
327.3
5.85
208
Aluminum
660
3.97 x 105
94.8
1.14 x 107
2 720
Silver
960.80
8.82 x 104
21.1
2 193
2.33 x 10°
558
1 063.00
1 083
Gold
6.44 X 10
15.4
2 660
1.58 X 10
377
Соpper
1.34 x 10
1 187
5.06 X 10
1 210
32.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Horizons: Exploring the Universe (MindTap Course …
Physics
ISBN:
9781305960961
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning