Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 67A
Related questions
Question
Molar masses for all compounds in the equation;
K: 39.10 g/mol
H2O: 18.02 g/mol
KOH: 56.11 g/mol
H2: 2.02 g/mol
2K (s) + 2 H2O (l) -----> 2 KOH (s) + H2 (g)
You need to plan a reaction to produce 20 L of H2. What mass of potassium K will you to accomplish this?
solve like the exmaple attached starting with the first step.
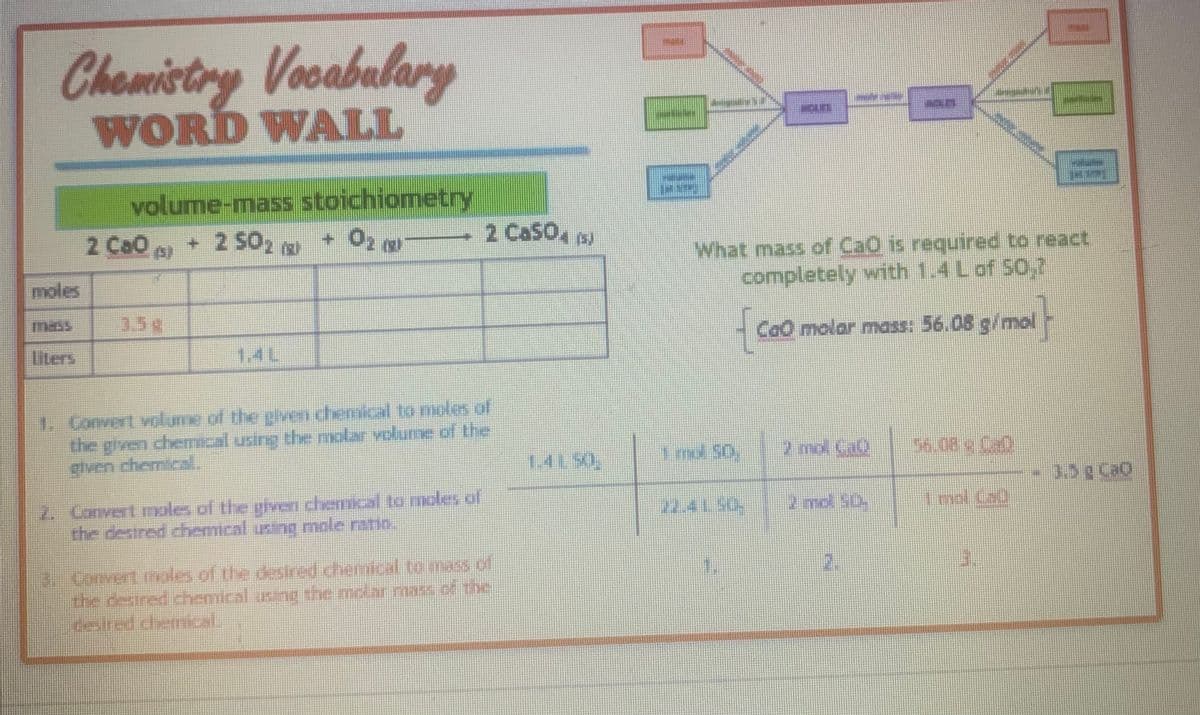
Transcribed Image Text:Chemistry Vocabulary
WORD WALL
moles
volume-mass stoichiometry
+ 2 502 0
+ 02 (2)
100 (3)
2 Ca50,
1. Convert volume of the given chemical to moles of
the given chemical using the molar volume of the
given chemical.
2. Convert males of the given chemical to moles of
the desired chemical using mole ratio.
3. Corvert nales of the desired chemical to mass of
the desired chemical using the molar masa of the
PAPJOVI
DLY
G
HELER
120
What mass of CaO is required to react
completely with 1.4 L of 50,7
{
CaO molar mass: 56.08 g/mol
ALAT
2 md. (10
2 mol 50.
56.08 % 0.0.
1
I mal lau
sau
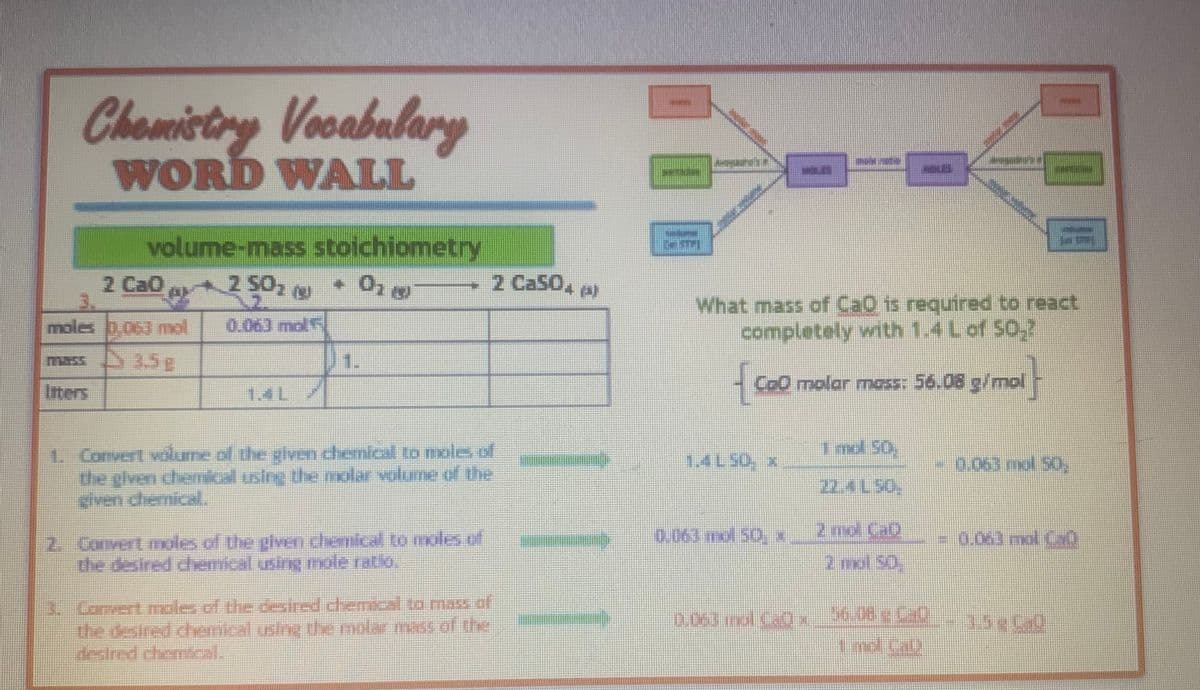
Transcribed Image Text:Chemistry Vocabulary
WORD WALL
volume-mass stoichiometry
tao
2 50₂ (
+ 01 8
moles 0,063 mol 0.06) mdf
mass 3.5c
Inters
1JL
1. Convert volume of the given chemical to moles of
the given chemical using the molar volume of the
2. Convert moles of the given chemical to moles of
the desired chemical using mole ratio.
2 Ca504 A)
3. Convert mole of the desired chemical to mass of
the desired chemical using the molar mass of the
destred chemical.
6
T
AUTOM
HARTULANTE
What mass of CaQ is required to react
completely with 1.4 L of SO₂?
{co
CoO molar mass: 56.08 g/mol
0.063 no 50 x
0.063 mol Cap x
1 mol 50.
22- L 50.
2 md. C.D
2nd 50
0.063 mol 50,
- CO
0,063 mol (30)
15:00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co