your answer in the provided box. A 409-g piece of copper tubing is heated to 89.5°C and placed in an insulated vessel containing 159 water at 22.8°C. Assuming no loss of water and a heat capacity for the vessel of 10.0 J/oC, what is the final temperature of the system (c of copper 0.387 J/g.0C)? g of 11 C
your answer in the provided box. A 409-g piece of copper tubing is heated to 89.5°C and placed in an insulated vessel containing 159 water at 22.8°C. Assuming no loss of water and a heat capacity for the vessel of 10.0 J/oC, what is the final temperature of the system (c of copper 0.387 J/g.0C)? g of 11 C
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
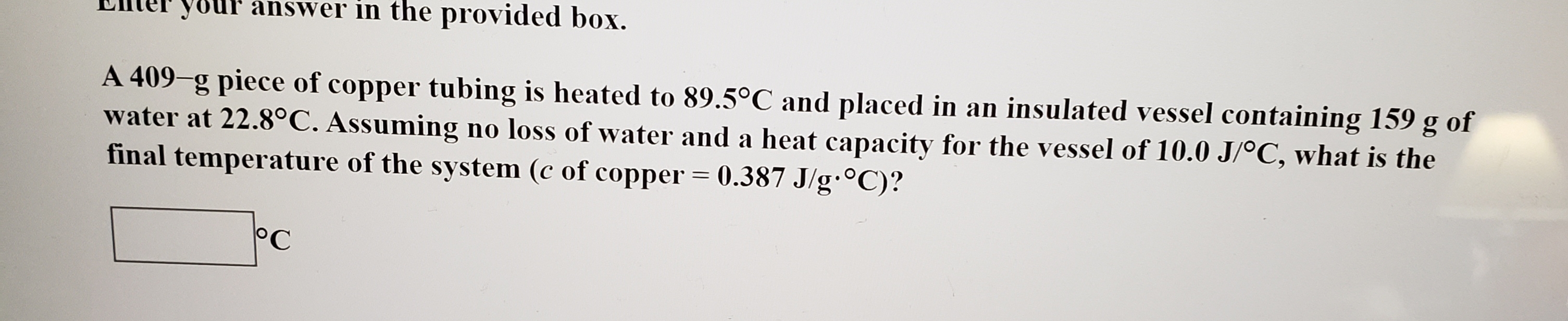
Transcribed Image Text:your answer in the provided box.
A 409-g piece of copper tubing is heated to 89.5°C and placed in an insulated vessel containing 159
water at 22.8°C. Assuming no loss of water and a heat capacity for the vessel of 10.0 J/oC, what is the
final temperature of the system (c of copper 0.387 J/g.0C)?
g of
11
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning