
Concept explainers
- a. Draw the resonance forms for SO2 (bonded O—S—O).
- b. Draw the resonance forms for ozone (bonded O—O—O).
- c. Sulfur dioxide has one more resonance form than ozone Explain why this structure is not possible for ozone.
(a)
To determine: The resonating structures of
Interpretation: The resonating structures of
Concept introduction: Resonance is the process in which a molecule gets different structures to define its bonding within the molecule. Such molecules cannot be represented in single Lewis structures. Resonating structures of such molecules are called contributing structures. In the process of resonance, shifting of lone pairs occurs with the bonds and other lone pairs.
Answer to Problem 1.26SP
The resonating structures of
Explanation of Solution
The resonating structures of the given molecule are shown as,
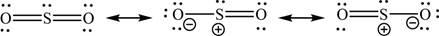
Figure 1
The given compound is
(b)
To determine: The resonating structures of
Interpretation: The resonating structures of
Concept introduction: Resonance is the process in which a molecule gets different structures to define its bonding within the molecule. Such molecules cannot be represented in single Lewis structures. Resonating structures of such molecules are called contributing structures. In the process of resonance, shifting of lone pairs occurs with the bonds and other lone pairs.
Answer to Problem 1.26SP
The resonating structures of the given molecule are shown in Figure 2.
Explanation of Solution
The resonating structures of the given molecule are shown as,

Figure 2
The given compound is trioxygen. In this molecule, three oxygen atoms are joined together by one double and one single bond. One oxygen atom carries a positive charge and one carries a negative charge. The positive charge, negative charge and a double bond present in conjugation in the given molecule. It results in the resonating structures of
(c)
To determine: The reason corresponding to the fact that ozone has only two resonating structures, whereas sulfur dioxide has three.
Interpretation: The reason corresponding to the given fact is to be stated.
Concept introduction: Resonance is the process in which a molecule gets different structures to define its bonding within the molecule. Such molecules cannot be represented in single Lewis structures. Resonating structures of such molecules are called contributing structures. In the process of resonance, shifting of lone pairs occurs with the bonds and other lone pairs.
Answer to Problem 1.26SP
The reason corresponding to the given fact is stated below.
Explanation of Solution
Both the sulfur dioxide and ozone molecules are triatomic and both contain atoms of oxygen family. But the resonating structures of sulfur dioxide are more than ozone. The main reason for this is the difference in the group number of the central atom.
In sulfur dioxide, sulfur is present as a central atom, whereas in ozone oxygen atom is present as a central atom. Rest of the surrounding atoms in both the molecules are same. Atomic number of sulfur is sixteen. Whereas, oxygen has the atomic number eight. Therefore, sulfur contains third shell too and it is capable to handle extra bond by using empty “d” orbital and also able to show more resonating structures. This is not the case with ozone molecule. Hence, sulfur dioxide has more resonating structures than ozone molecule.
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry (9th Edition)
Additional Science Textbook Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
General Chemistry: Atoms First
Chemistry: Structure and Properties (2nd Edition)
Chemistry: A Molecular Approach (4th Edition)
Organic Chemistry
Essential Organic Chemistry (3rd Edition)
- Part 1 What do each of the following sets of compounds/ions have in common with each other? a) SO3, NO3-, CO32- b) O3,SO2, NO2- Part 2 What do each of the following sets of compounds/ions have in common with each other? a) XeCl4, XeCl2 b) ICl5, TeF4, ICl3, PCl3, SCl2, SeO2arrow_forwardChemistry (a) Write three more resonance structures for each of compounds 1 and 2. (b) In each of compounds 1 and 2, determine which resonance structure contributes the most and explain your answer. (c) Are the 3/4 structures resonance structures or different compounds? Same question for 5/6 structures. Explain your answers.arrow_forwardDraw Lewis electron dot structures for CH3Cl (methyl chloride, a topical anesthetic), H2O2 (hydrogen peroxide, with an O-O bond), and NH2OH (with an N-O bond).arrow_forward
- If we were taking care of the double bond on oxygen to two people what would the best comparison be? A. Two people who are pulling their money together to buy a car for themselves to drive? B to unhappy people sitting in a restaurant who always returns their dinner to the kitchen? C. Two people in a committed relationship but who might separate if they encounter enough stress? D. Two people who want to go out on a date together but cannot decide what movie to watch?arrow_forwardAtom A has 4 valence electrons. Atom Z has 6 valence electrons. For the AZ3-2 ion How many valence electrons are in the structure? b. How many single bonds are in the structure? c. How many double bonds are in the structure? d. How many triple bonds are in the structure? e. How many lone pairs are on the central atom in the structure? f. What is the shape of the structure? g. What are the bond angles of this ion?arrow_forwardDraw 2 best resonance structures, and circle the best resonance structure(s) of the ones you providedarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning



