
Concept explainers
Consider the following flat drawing of methane
a. What is
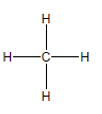
b. Are the electron domains of this flat
c. Use model materials to make a model of
d. In which representation, the drawing above or the model in your hand (circle one) are theH’s of
e. Confirm that your model looks like the following drawing. The wedgebond represents a bond coming out of the page, and the dash bondrepresents a bond going into the page
f. You will often see methane drawn as if it were flat (like on the previous page). Why is thismisleading, and what is left to the viewer’s imagination when looking at such a drawing?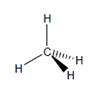
Trending nowThis is a popular solution!

Chapter 1 Solutions
Organic Chemistry: A Guided Inquiry
Additional Science Textbook Solutions
General, Organic, & Biological Chemistry
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry
Chemistry: The Central Science (13th Edition)
Fundamentals of Heat and Mass Transfer
- In addition to CO, CO2, and C3O2, there is another molecular oxide of carbon, pentacarbon dioxide, C5O2, a yellow solid. (a) What is the approximate C-to-C-to-O bond angle in pentacarbon dioxide? (b) What is the approximate C-to-C-to-C bond angle in this compound?arrow_forwardElectrons in bonds are negatively charged and therefore repel each other. If you had a molecule of formula AB 2 , with A being the central atom, what would you predict the 3D shape to be to allow maximum distance between the two B atoms bonded to A? Linear shape, with a B atom on each side of A and a bond angle of 80 degrees A bent or V shape, with a B - A - B bond angle less than 180°arrow_forwardDoes BeCl2 have a dipole moment?If so, in which direction does the net dipole point?arrow_forward
- Give approximate values for the indicated bond angles:(a) Cl—S—Cl in SCl2(b) N—N—O in N2O(c) Bond angles 1, 2, and 3 in vinyl alcohol (a component of polymers and amolecule found in outer space)arrow_forwardWhat exactly is the difference between (E)/(Z) and cis/trans nomenclature? In my class, we usually use (E)/(Z) for double bonds- is it possible to use it for single bonds? Is (E)/(Z) used when cis/trans is not able to depict the geometric bonding as there are four different groups?arrow_forwardElectrons in bonds are negatively charged and therefore repel each other. If you had a molecule of formula AB 4 , with A being the central atom, what would you predict the 3D shape to be to allow maximum distance between the four B atoms bonded to A? Some kind of tridimensional shape with A in the middle and B-A-B bond angles bigger than 90° A bidmensional shape like a "" sign where the B - A - B bond angles ate exactly 90 degreesarrow_forward
- 4.Examine the model and real structures for SO2. A.In what way does the central atom violate the octet rule? When is it possible for molecules to have this exception to the octet rule? B.Calculate the difference in the bond angle between the real and model structure? Is this difference larger or smaller than the difference observed for H2O? Why is the deviation between the real and model structure different for SO2compared to H2O? The deviation for H2O is 5 degreesarrow_forwardDepending on the protein under study, the bond angle of a drug molecule can become critical to successfully deactivating a viral protein. For this reason, chemists are frequently concerned with the 3D shape of their molecules and their bond angles. The oxygen atom shown in the structure has a tetrahedral electronic geometry, meaning we would predict that it would have 109.5° bond angles. However, the actual structure, the bond angles are smaller than 109.5°. Explain why this compression occurs. Make sure to discuss what’s happening around/what groups are present around the oxygen atom.arrow_forward1 (a.) Should all of the angles in methane (CH4) be equal? b.) What additional information does the VSEPR theory give you beyond electron dot structures?arrow_forward
- The curved arrow notation introduced in Section 1.6B is a powerful method used by organic chemists to show the movement of electrons not only in resonance structures, but also in chemical reactions.Because each curved arrow shows the movement of two electrons, following the curved arrows illustrates what bonds are broken and formed in a reaction. Consider the following three-step process. (a) Add curved arrows in Step [1] to show the movement of electrons. (b) Use the curved arrows drawn in Step [2] to identify the structure of X. X is converted in Step [3] to phenol and HCl.arrow_forwardBuild a model of methane – - What are the approximate bond lengths and bond angles in methane?- What is the name given to the shape of methane?- Explain what is meant by the term ‘sp3 hybridisation’.- Use wedge and dash bonds to draw the shape of the model you have made.arrow_forwardWhat are the bond angles around the carbon and oxygen atoms? A) C: 109.5 O: 104.5 B) C: 104.5 O: 109.5 C) C: 120 O: 104.5 D) C: 109.5 O: 180arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





