
Concept explainers
(a)
Interpretation:
To label each bond in the compounds as ionic or covalent.
Concept introduction:
In chemistry
Ionic bond is the bond that is formed when one or more electrons from the valence shell of an atom are completely transfer to another atom valence shell. Formation of ionic bond is shown below in Fig.1.
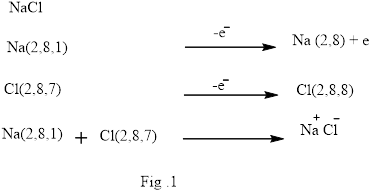
Covalent bond is formed between two atoms by mutual sharing of electrons pairs between them. By mutual sharing of electron pairs the atom attains the stable noble gas configuration. Thus in the covalent bonding the combining atom have equal claim of the shared electron pair. Illustration of covalent bond is shown below in Fig.2.
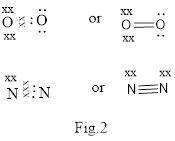
(b)
Interpretation:
To label each bond in the compounds as ionic or covalent.
Concept introduction:
In chemistry chemical bond is defined as an attractive force that contains various constituent particles (atoms, ions and molecules) in different chemical species. The chemical bond is off many types. Ionic or electrovalent bond, covalent bond and coordinate covalent bond.
Ionic bond is the bond that is formed when one or more electrons from the valence shell of an atom are completely transfer to another atom valence shell. Formation of ionic bond is shown below in Fig.1.

Covalent bond is formed between two atoms by mutual sharing of electrons pairs between them. By mutual sharing of electron pairs the atom attains the stable noble gas configuration. Thus in the covalent bonding the combining atom have equal claim of the shared electron pair. Illustration of covalent bond is shown below in Fig.2.
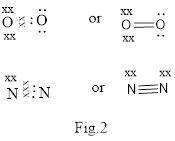
(c)
Interpretation:
To label each bond in the compounds as ionic or covalent.
Concept introduction:
In chemistry chemical bond is defined as an attractive force that contains various constituent particles (atoms, ions and molecules) in different chemical species. The chemical bond is off many types. Ionic or electrovalent bond, covalent bond and coordinate covalent bond.
Ionic bond is the bond that is formed when one or more electrons from the valence shell of an atom are completely transfer to another atom valence shell. Formation of ionic bond is shown below in Fig.1.

Covalent bond is formed between two atoms by mutual sharing of electrons pairs between them. By mutual sharing of electron pairs the atom attains the stable noble gas configuration. Thus in the covalent bonding the combining atom have equal claim of the shared electron pair. Illustration of covalent bond is shown below in Fig.2.
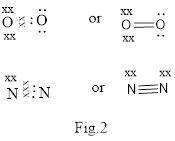
(d)
Interpretation:
To label each bond in the compounds as ionic or covalent.
Concept introduction:
In chemistry chemical bond is defined as an attractive force that contains various constituent particles (atoms, ions and molecules) in different chemical species. The chemical bond is off many types. Ionic or electrovalent bond, covalent bond and coordinate covalent bond.
Ionic bond is the bond that is formed when one or more electrons from the valence shell of an atom are completely transfer to another atom valence shell. Formation of ionic bond is shown below in Fig.1.

Covalent bond is formed between two atoms by mutual sharing of electrons pairs between them. By mutual sharing of electron pairs the atom attains the stable noble gas configuration. Thus in the covalent bonding the combining atom have equal claim of the shared electron pair. Illustration of covalent bond is shown below in Fig.2.
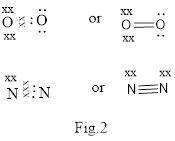
(d)
Interpretation:
To label each bond in the compounds as ionic or covalent.
Concept introduction:
In chemistry chemical bond is defined as an attractive force that contains various constituent particles (atoms, ions and molecules) in different chemical species. The chemical bond is off many types. Ionic or electrovalent bond, covalent bond and coordinate covalent bond.
Ionic bond is the bond that is formed when one or more electrons from the valence shell of an atom are completely transfer to another atom valence shell. Formation of ionic bond is shown below in Fig.1.

Covalent bond is formed between two atoms by mutual sharing of electrons pairs between them. By mutual sharing of electron pairs the atom attains the stable noble gas configuration. Thus in the covalent bonding the combining atom have equal claim of the shared electron pair. Illustration of covalent bond is shown below in Fig.2.
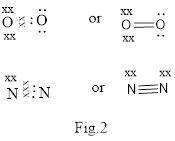
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
ORGANIC CHEMISTRY-STUDY GDE...-W/ACCESS
- Use the Lewis model to determine the formula for the compound that forms from each pair of atoms. Ba and Sarrow_forwardA. The Lewis diagram for POCl3 is: The electron-pair geometry around the P atom in POCl3 is ______ There are _____ lone pair(s) around the central atom, so the geometry of POCl3 is ______ B. The Lewis diagram for BH2- is: The electron-pair geometry around the B atom in BH2- is ______ There are _____ lone pair(s) around the central atom, so the geometry of BH2- is _____ (c) The amount of acrylamide found in potato chips is 1.7 mg/kg. If a serving of potato chips is 35 g, how many moles of acrylamide are you consuming? ________molarrow_forward1. How many electrons will an iodine atom donate or accept, based on its number of valence electrons? A. Donate 7 electrons B. Donate 1 electron C. Accept 7 electrons D. Accept 1 electrons 2.What type of bond is formed between the two nitrogen atoms in diatomic nitrogen, N2? A. Triple Bond B. Double Covalent Bond C. Double Ionic Bond D. Single Bond 3.Which metal would form a stronger metallic bond? A. Lithium B. Sodium C. Strontium D. Tungsten 4. What holds the metal ions together in a lattice? A. Hydrogen Bonds B. Covalent Bonds C. Metallic Bonds D. Ionic Bondsarrow_forward
- 1) What do you predict the average bond length and average bond energy values for the F-F bond based on the chart below? WIll it be higher than, lower than, or the same as the other bonds shown in the chart. Justify your answer. Bond Average Bond Length (PM) Average Bond Energy (KJ/MOLE) CL-CL 199 243 Br-Br 228 193 I-I 267 151 2) Explain how a stable covalent bond is formed in terms of attractive and repulsive forces. Note: Please briefly explain, Thank you.arrow_forwardhow many shared electrons make up a single covelant bond? what is the maximum number of covalent bonds that can be formed between any two atomsarrow_forwardWithin a covalent Lewis structure, what is the difference between lone pair and bonding pair electrons?arrow_forward
- In which of the molecular models in Figure 8 does the carbon atom have the largest partial positive charge? * A B C The charge is evenly distributed over each of the moleculesarrow_forwardA. Name the covalent compounds 1. B2Si 2. Cl2O5 3. PCl3 4. Cl2O 5. Si2Br6 6. F2O5 B. Name ionic compounds 1. NH4Cl 2. MgS 3. Fe(NO3)3 4. TiBr3 5. Cu3P 6. SnSe2 7. GaAs 8. Pb(SO4)2 9. Be(HCO3)2 10. Mn2(SO3)3 11. Al(CN)3 C. Using electronegative to determine polarity. (Include the equation and type of compound.)arrow_forwardDiscuss the formation of ionic and molecular compounds: Explain the types of bonds and ions involved where applicable. Identify and discuss at least 5 polyatomic ions used in compounds of medical significancearrow_forward
- All of the following are structural features that may be observed in Lewis structures of covalent molecules Except. a. Melting Points b. Triple Bonds c. Lone pairs of electrons d. Double Bond Explain why?arrow_forwardThe bond that forms between two oxygen atoms to form O2, oxygen gas ... a) involves shared electrons b) is a polar covalent bond c) is a non-polar covalent bond d) a and b e) a and carrow_forward1. Which figure show compound whose atoms are bonded by covalent bond? 2. What do we call to the formula represented by figures A and B? 3. What type of compound is in figure A?arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





