
Concept explainers
Assign formal charges to each
a. 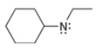 b.
b. 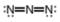 c.
c. 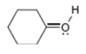 d.
d. 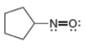
(a)
Interpretation: The formal charge to each
Concept introduction:
The formal charge on an atom is calculated by the formula,
Answer to Problem 1.40P
In the given molecule, the formal charge on nitrogen atom is
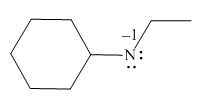
Explanation of Solution
The given molecule is,
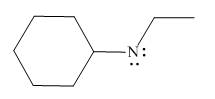
Figure 1
The formal charge on an atom is calculated by the formula,
For the nitrogen atom,
Substitute these values in above equation to calculate the formal charge on nitrogen atom.
Thus, in the given molecule, the formal charge on nitrogen atom is

Figure 2
In the given molecule, the formal charge on nitrogen atom is
(b)
Interpretation: The formal charge to each
Concept introduction: The formal charge on an atom is calculated by the formula,
Answer to Problem 1.40P
The formal charge to each
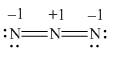
Explanation of Solution
The given molecule is,
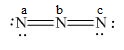
Figure 3
Here, a, b, and c are used to indicate nitrogen atoms.
The formal charge on an atom is calculated by the formula,
For the nitrogen atom,
Substitute these values in the above equation to calculate the formal charge on
In the given molecule, bond pairs and lone pairs in
Thus, the formal charge on
For the nitrogen atom,
Substitute these values in the above equation to calculate the formal charge on
Thus, the formal charge on
Hence, the formal charge to each
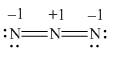
Figure 4
In the given molecule, the formal charge on nitrogen atom of both
(c)
Interpretation: The formal charge to each
Concept introduction: The formal charge on an atom is calculated by the formula,
Answer to Problem 1.40P
In the given molecule, the formal charge on oxygen atom is
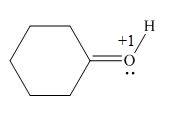
Explanation of Solution
The given species is,
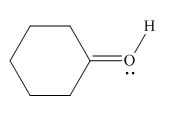
Figure 5
The formal charge on an atom is calculated by the formula,
For the oxygen atom,
Substitute these values in above equation, to calculate the formal charge on oxygen atom.
Thus, in the given molecule, the formal charge on oxygen atom is

Figure 6
In the given molecule, the formal charge on oxygen atom is
(d)
Interpretation: The formal charge to each
Concept introduction: The formal charge on an atom is calculated by the formula,
Answer to Problem 1.40P
In the given molecule, the formal charge on both nitrogen and oxygen atom is zero as shown below.
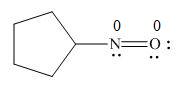
Explanation of Solution
The given species is,
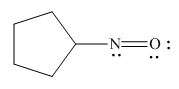
Figure 7
The formal charge on an atom is calculated by the formula,
For the oxygen atom,
Substitute these values in above equation, to calculate the formal charge on oxygen atom.
For the nitrogen atom,
Substitute these values in above equation, to calculate the formal charge on nitrogen atom.
Thus, in the given molecule, the formal charge on both nitrogen and oxygen is zero as shown below.

Figure 8
In the given molecule, the formal charge on both nitrogen and oxygen is zero.
Want to see more full solutions like this?
Chapter 1 Solutions
Package: Organic Chemistry with Connect 2-year Access Card
Additional Science Textbook Solutions
General Chemistry: Atoms First
Organic Chemistry
Chemistry In Context
Chemistry: Structure and Properties (2nd Edition)
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
- In the Lewis structure for chloromethane, the chlorine atom is sharing _____ electron pair and “owns” _____ of those electrons. Also, the chlorine atom possesses two electrons from each of _____ unshared pairs. The total number of electrons that belong to chlorine is 7 . Chlorine is a Group ____ element. The formal charge on chlorine in chloromethane is ____.arrow_forwardOn the basis of the electronegativity values given in Fig. 12.3, indicate whether each of the following bonds would be expected to be ionic, covalent, or polar covalent. msp;a.HOc.HHb.OOd.HClarrow_forwardWhich is not a valid resonance structure for the following molecule? Explain your answer. It might be useful to draw the lone pairs on the molecule.arrow_forward
- Lewis structure of H2S. Any polar bonds in molecule? Polar or non polar?arrow_forwardConsidering the structure on the far left as the original resonance structure, which of the subsequent resonance structures (A-D) is implausible. Explain why you believe the resonance structure to be implausible.arrow_forwardLewis structure of HCN. Any polar bonds in molecule? Polar or non polar ?arrow_forward
- Create your own Lewis structure molecule using the the molecular model kitarrow_forwardConsider compounds A–D, which contain both a heteroatom and a double bond. (a) For which compounds are no additional Lewis structures possible? (b) When two or more Lewis structures can be drawn, draw all additional resonance structures.arrow_forwardAssign formal charges to each N and O atom in the given molecules. All lone pairs have been drawn in.arrow_forward
- Based on the photo, draw a bond line structure that is correct for the following Lewis structure?arrow_forwardi wanna help to complete this table (Number of Valence electrons,Lewis Structure and Formal charge on central atom) table in attachmentsarrow_forwardwhich of the pair is more stable, resonance structure if neededarrow_forward
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





