
Concept explainers
Assign formal charges to each carbon atom in the given species. All lone pairs have been drawn in.
a.
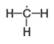 d.
d. 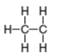
(a)
Interpretation: The formal charge on each carbon atom in the given species is to be assigned.
Concept introduction: The formal charge on an atom is calculated by the formula,
Answer to Problem 1.41P
In the given species, the formal charge on carbon atom of
Explanation of Solution
The given species is
The formal charge on an atom is calculated by the formula,
Substitute these values in the above equation to calculate the formal charge.
For the carbon present in
Substitute these values in above equation to calculate the formal charge on the carbon of
Thus, in the given species, the formal charge on carbon atom of
(a) In the given species, the formal charge on carbon atom of
(b)
Interpretation: The formal charge on each carbon atom in the given species is to be assigned.
Concept introduction: The formal charge on an atom is calculated by the formula,
Answer to Problem 1.41P
In the given species, the formal charge on carbon atom is zero as shown below.
Explanation of Solution
The given species is
The formal charge on an atom is calculated by the formula,
Substitute these values in above equation to calculate the formal charge on carbon atom.
Thus, in the given species, the formal charge on carbon atom is zero.
In the given species, the formal charge on carbon atom is zero.
(c)
Interpretation: The formal charge on each carbon atom in the given species is to be assigned.
Concept introduction: The formal charge on an atom is calculated by the formula,
Answer to Problem 1.41P
In the given species, the formal charge on carbon atom is zero as shown below.
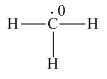
Explanation of Solution
The given species is,
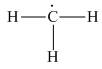
Figure 1
The formal charge on an atom is calculated by the formula,
For the given carbon atom,
Substitute these values in above equation, to calculate the formal charge on carbon atom.
Thus, in the given species, the formal charge on carbon atom is zero.

Figure 2
In the given species, the formal charge on carbon atom is zero.
(d)
Interpretation: The formal charge on each carbon atom in the given species is to be assigned.
Concept introduction: The formal charge on an atom is calculated by the formula,
Answer to Problem 1.41P
In the given species, the formal charge on carbon atom of
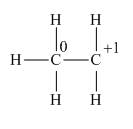
Explanation of Solution
The given species is,
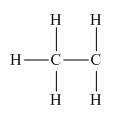
Figure 3
The formal charge on an atom is calculated by the formula,
Substitute these value in above equation to calculate the formal charge on the carbon of
For the carbon of
Substitute these value in above equation to calculate the formal charge on the carbon of
Thus, in the given species, the formal charge on carbon atom of

Figure 4
In the given species, the formal charge on carbon atom of
Want to see more full solutions like this?
Chapter 1 Solutions
Connect Access Card For Organic Chemistry
Additional Science Textbook Solutions
Chemistry: The Molecular Nature of Matter
Organic Chemistry (9th Edition)
Organic Chemistry
General, Organic, and Biological Chemistry (3rd Edition)
Principles of General, Organic, Biological Chemistry
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
- Write the chemical formula and Lewis structure of the following each of which contains five carbon atoms: (a) an alkane. (b) an alkene. (c) an alkynearrow_forward1.a)draw the complete Lewis structure (atoms, dots, lines, dashes, wedges, formal charges) for the formula OF2 b) draw the complete Lewis structure (atoms, dots, lines, dashes, wedges, formal charges) for the formula CH2Cl2arrow_forwardWhich type of bonds does methane or CH4 have, and why does one carbon atom bond with four hydrogen atoms? is there a way to explain in terms of valence electrons and electronegativity.arrow_forward
- Write Lewis structures for each molecule or ion. Include resonance structures if necessary and assign formal charges to all atoms. If necessary, expand the octet on the central atom to lower formal charge.a. SO42 - b. HSO4- c. SO3 d. BrO2-arrow_forward1. a) draw the complete Lewis structure (atoms, dots, lines, dashes, wedges, formal charges) for the formula SeBr4 b)draw the complete Lewis structure (atoms, dots, lines, dashes, wedges, formal charges) for the formula SO3arrow_forwardAdd lone pairs to the N and O atoms to give octets and then determine the shape around each indicated atom.arrow_forward
- Formaldehyde, CH2O, is used as an embalming agent. Draw the structure of CH2O, including lone pairs.arrow_forward1. In its Lewis dot symbol, which element would have four (4) dots? Select one: a.Si b.F c.N d.S 2. Which compound is expected to be soluble in a polar solvent? Select one: a.CH4 b.NH3 c.None of these. d.BF3 3. Which compound is nonpolar? Select one: a.CO2 b.None of these. c.HCl d.H2O 4. In HCN, the formal charge on nitrogen is Select one: a.+1 b.0 c.–1 d.None of these. 5. For the carbonate anion, CO3–2, the number of contributing equienergetic resonance structures is Select one: a.3 b.4 c.1 d. 2arrow_forwardThe n-propyl cation can be formed from a molecule such as When the C–Cl bond is broken so that both electrons leave with Cl, the fragments formed are The carbon atom that had been attached to Cl is now sharing ____ electron pairs. In each shared pair the carbon atom owns ____ electron. The number of electrons that belong to carbon is ____. The formal charge on the carbon atom is ____. The correct Lewis structure for the n-propyl cation isarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





