
Concept explainers
(a)
Interpretation:
To draw a valid Lewis structure for the given compound
Concept introduction:
In chemistry Lewis structure is very important as shown below in the Fig.1
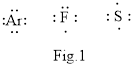
The above shown atoms combine to form molecule to attain stale noble gas configuration.
The structure in which all the electrons in the valence shells of the combining atoms are shown in the form of dots is called Lewis structure. The valence electrons that take in the bonding are called the bonding electrons and the valence electrons that are not participate is the nonbonding electrons.
(b)
Interpretation:
To draw a valid Lewis structure for the given compound
Concept introduction:
In chemistry Lewis structure is very important as shown below in the Fig.1
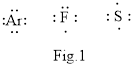
The above shown atoms combine to form molecule to attain stale noble gas configuration.
The structure in which all the electrons in the valence shells of the combining atoms are shown in the form of dots is called Lewis structure. The valence electrons that take in the bonding are called the bonding electrons and the valence electrons that are not participate is the nonbonding electrons.
(C)
Interpretation:
To draw a valid Lewis structure for the given compound
Concept introduction:
In chemistry Lewis structure is very important as shown below in the Fig.1
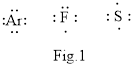
The above shown atoms combine to form molecule to attain stale noble gas configuration.
The structure in which all the electrons in the valence shells of the combining atoms are shown in the form of dots is called Lewis structure. The valence electrons that take in the bonding are called the bonding electrons and the valence electrons that are not participate is the nonbonding electrons.
(d)
Interpretation:
To draw a valid Lewis structure for the given compound
Concept introduction:
In chemistry Lewis structure is very important as shown below in the Fig.1
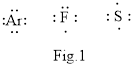
The above shown atoms combine to form molecule to attain stale noble gas configuration.
The structure in which all the electrons in the valence shells of the combining atoms are shown in the form of dots is called Lewis structure. The valence electrons that take in the bonding are called the bonding electrons and the valence electrons that are not participate is the nonbonding electrons.
(e)
Interpretation:
To draw a valid Lewis structure for the given compound
Concept introduction:
In chemistry Lewis structure is very important as shown below in the Fig.1
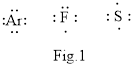
The above shown atoms combine to form molecule to attain stale noble gas configuration.
The structure in which all the electrons in the valence shells of the combining atoms are shown in the form of dots is called Lewis structure. The valence electrons that take in the bonding are called the bonding electrons and the valence electrons that are not participate is the nonbonding electrons.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
KCTCS Organic Chemistry Value Edition (Looseleaf) - Text Only
- Considering structures A–D, classify each pair of compounds as isomers, resonance structures, or neither: (a) A and B; (b) Aand C; (c) A and D; (d) B and Darrow_forwardDraw complete Lewis structures for the following condensed structural formulas.(a) CH3(CH2)3CH(CH3)2 (b) (CH3)2CHCH2Cl(c) CH3CH2COCN (d) CH2CHCHO(e) (CH3)3CCOCHCH2 (f) CH3COCOOHarrow_forwardWhich structure is correct?arrow_forward
- Make a model of propane (C3H8), and draw this model using dashed lines and wedges to represent bonds going back and coming forwardarrow_forwardHow can you convert CsCl into NaCl like structure?arrow_forwardConsidering structures A–D, classify each pair of compounds as isomers, resonance structures, or neither: (a) A and B; (b) A and C; (c) A and D; (d) B and D.arrow_forward
- give some tips how to find significant resonance structures of organic moleculesarrow_forwardDraw and upload a Lewis structure for CH4. as well as answer the following questions. -what is the hybrizatjon around the carbon? - what is the electron pair geometry for your drawing? -what is the bond angle between the C-H bonds of CH4 what is the molecular shape for CH4?arrow_forwardWhich bond is more polar? And what is the direction of the polarity in each?arrow_forward
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

