
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 14CTQ
A student draws the picture of ammonia
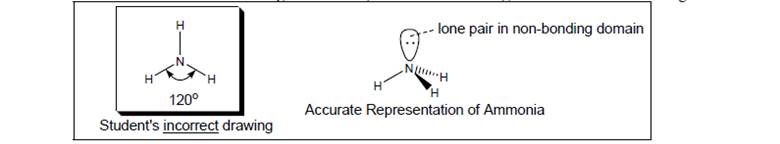
a. What did the student omit from his drawing?
b. What is the actual
c. Explain why water, ammonia, and methane (shown below) all have about the same bondangles (close to 109.5°) even though they have different numbers of bonds.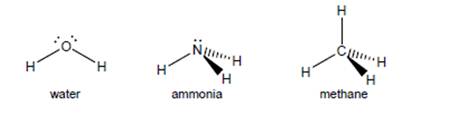
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
PI3Br2 is a nonpolar molecule. Based on this information, determine the I−P−I bond angle, the Br−P−Br bond angle, and the I−P−Br bond angle.
Enter the number of degrees of the I−P−I, Br−P−Br, and I−P−Br bond angles, separated by commas (e.g., 30,45,90)
Atom A has 4 valence electrons. Atom Z has 6 valence electrons.
For the AZ3-2 ion
How many valence electrons are in the structure?
b. How many single bonds are in the structure?
c. How many double bonds are in the structure?
d. How many triple bonds are in the structure?
e. How many lone pairs are on the central atom in the structure?
f. What is the shape of the structure?
g. What are the bond angles of this ion?
Good hand written explanation
Asap
A molecule of chloroform, CHCl3, has the same shape as a molecule of methane, CH4. However, methane’s boiling point is -164 degreeC and chloroform’s boiling point is 62 degree C. Explain the difference in boiling points, using 3D Lewis Structures to aid your simple explanation.
Chapter 1 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 1 - (E) What does the number (+Z) at the center of...Ch. 1 - Prob. 2CTQCh. 1 - Prob. 3CTQCh. 1 - Prob. 4CTQCh. 1 - Prob. 5CTQCh. 1 - Prob. 6CTQCh. 1 - Prob. 7CTQCh. 1 - You hear a student from a nearby group say that...Ch. 1 - Use VSEPR to explain why the HBH bond angle of BH3...Ch. 1 - Both the HCH and HCO bond angles of H2CO...
Ch. 1 - Prob. 11CTQCh. 1 - Consider the following flat drawing of methane...Ch. 1 - Use VSEPR to assign a value of (close to) 109.5,...Ch. 1 - A student draws the picture of ammonia (NH3) in...Ch. 1 - Prob. 15CTQCh. 1 - How many central atoms does the molecule H2NCH3...Ch. 1 - Indicate the bond angle and shape about each...Ch. 1 - Explain how there can be two kinds of bent:...Ch. 1 - A student makes the following statement: “The...Ch. 1 - A student who missed this class needs to know how...Ch. 1 - Prob. 1ECh. 1 - Prob. 2ECh. 1 - Consider the incomplete valence shell...Ch. 1 - How many valence electrons does a neutral a. K...Ch. 1 - Consider the molecules AlCl3 (aluminum chloride)...Ch. 1 - Draw an example of a bent molecule with a bond...Ch. 1 - Label each atom marked with an arrow with the...Ch. 1 - a model of each of the following molecules: a....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- On the left side of Figure 3.6, label the areas shown with a dotted line where... one bond can form. one bond can form.arrow_forwarda Draw the Lewis structure for CH4 in the window below and then decide if the molecule is polar or nonpolar.arrow_forwardDraw the lewis structure of SO2 ( best resonance) and 2nd best resonance showing the shape and bond angles of each one, the 3D structure with polar bonds or bonds dipole of each one and the molecular polarity. Here is an example of what I want the answer to be like:arrow_forward
- 2. Do you think the electrons are shared in a similar way in compounds Br2 and HBr? In other words, do they have the same type of bonds?arrow_forward1.You have made structures of NH3 and H2CO molecules in the Part A of your lab report.Both NH3 and H2CO molecules have three electron groups around the central atom.However, their molecular geometries are not the same. Explain this difference. 2. Draw the Lewis electron dot structure for SCl2 and discuss its molecular geometry. IsSCl2 molecule polar or nonpolar? Explain your answer.arrow_forward1. Consider the diatomic molecules ( molecules with 2 atoms ) in this exercise : O2, N2, Cl2, and HCl . Do you think a diatomic molecule can be any shape other than linear ? Explain . because They are only two alems 2. Look at the Lewis structure you drew for SCl2 and CS2 Both of these molecules are made up of three atoms but have different molecular geometries . a . How is the arrangement of electrons around the central atoms different ? Give the number of lone pairs and bonding groups around each central atom Based on your previous answer , explain why SCl2 can't be linear . c . Explain why one molecule is polar and the other is nonpolararrow_forward
- For each of the Lewis structures shown below, predict the Electron Geometry, Molecular Geometry and Bond Angle. Lastly, using the same format as shown in the last column of Table 1, draw a sketch (using wedges and dashes to show 3D if needed) of the Molecular Geometry.arrow_forwardHow many σ-bonds are in the molecule? σ-bonds How many π-bonds are in the molecule? π-bonds For bond angles, respond with 60o, 90o, 109o, 120o, or 180o (do not include the degree sign in your response). What is the approximate H-C-C bond angle in the molecule? o What is the approximate C-O-H bond angle in the molecule? o What is the approximate C-N-H bond angle in the molecule? o What is the approximate O=C-O bond angle in the molecule? oarrow_forwardHow many LONE pairs of electron does ONE water molecule have? a. two b. three c. one d. none I need explanation in a research way. Please don't base the answer to any articles that can be seen in google instead base it with a research paper or any studies with authors. Book-supported is also okay.arrow_forward
- The bond length of NaF is 231.00 pm. What would be the dipole moment, in D, of this compound by assuming a completely ionic bond? Remember e = 1.60x10-19 C; 1 D = 3.34x10-30 C*marrow_forwardDraw the attraction between a water molecule and a molecule of NF3 First, draw one molecule and add the partial charges where needed - use the ΔEN to determine the types of bonds. Then, draw the second molecule so that the δ+ on one molecule lines up across from the δ- on the other. Since we can't draw the molecules here you will answer questions about the drawings that you made on the homework worksheet. NF3 has ____________ bonds with a ΔEN = ____________ . NF3 has 4 REDs and is symmetrical/asymmetrical (answer is ________ ) making it a polar/nonpolar (answer is __________ ) molecule. Each F has a ______________ charge and the N has a ________ charge. H2O has 2 ___________ bonds with a ΔEN = ___________ . H2O has 4 REDs and is symmetrical/asymmetrical (answer is ___________ . ) making it a polar/nonpolar (answer is _________ ) molecule. Each H has a _____________ charge and the O has a ____________ charge. The strongest possible attractive force between these two…arrow_forwardDraw the attraction between a water molecule and a molecule of PCl3 First, draw one molecule and add the partial charges where needed - use the ΔEN to determine the types of bonds. Then, draw the second molecule so that the δ+ on one molecule lines up across from the δ- on the other. Since we can't draw the molecules here you will answer questions about the drawings that you made on the homework worksheet. PCl3 has ________ bonds with a ΔEN = ________ . PCl3 has 4 REDs and is symmetrical/asymmetrical (answer is ___________ ) making it a polar/nonpolar (answer is ___________ ) molecule. Each Cl has a __________ charge and the P has a __________ charge. H2O has 2 ________ bonds with a ΔEN = +__________ . H2O has 4 REDs and is symmetrical/asymmetrical (answer is _________ ) making it a polar/nonpolar (answer is __________ ) molecule. Each H has a ___________ charge and the O has a _________ charge. The strongest possible attractive force between these two molecules…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY