
(a)
Interpretation:
To predict the hybridization and geometry around each indicated atom.
Concept introduction:
Molecular geometry is the three dimensional shape that a molecule in space. It is determine by considering the central atom and the surrounding atom and electron pairs. The shape of the molecule is determined by using Valence Shell Electron Pair Repulsion method. Some of the most common shapes that can be determined by this method are linear, tetrahedral, trigonal planar and pyramidal.
For example.,
Linear (angle = 180o)
Trigonal planar (angle = 120o)
Tetrahedral (angle = 109.5o)
Hybridization is the concept of mixing atomic orbital into new hybrid orbitals suitable for the electron pairing to form
Answer to Problem 1.69P
The hybridization and geometry of
![]()
is sp3 and tetrahedral
Explanation of Solution
For the hybridization, count the number of groups present around each atom. For example 4 groups = sp3, 3 groups = sp2, 2 groups = sp. And for the geometry count the surrounding atoms and lone pairs.
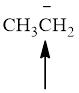
Fig.1
In the given compound (Fig.1), the central atom is carbon. It is surrounding by 3 atoms and a lone pair. So the geometry is tetrahedral. Number of groups present around the carbon atom is 4 so the hybridization is sp3.
The geometry is tetrahedral and the hybridization is sp3.
(b)
Interpretation:
To predict the hybridization and geometry around each indicated atom.
Concept introduction:
Molecular geometry is the three dimensional shape that a molecule in space. It is determine by considering the central atom and the surrounding atom and electron pairs. The shape of the molecule is determined by using Valence Shell Electron Pair Repulsion method. Some of the most common shapes that can be determined by this method are linear, tetrahedral, trigonal planar and pyramidal.
For example.,
Linear (angle = 180o)
Trigonal planar (angle = 120o)
Tetrahedral (angle = 109.5o)
Hybridization is the concept of mixing atomic orbital into new hybrid orbitals suitable for the electron pairing to form chemical bonds and valence bonds in other words mixing of two new orbital having same energy and shape. The orbital is called the hybrid orbital and the process is the hybridization. For example mixing s-orbital and p-orbital to form new hybridization is called sp-hybridization.
Answer to Problem 1.69P
The hybridization and geometry of
is nitrogen = sp3 and tetrahedral
Explanation of Solution
For the hybridization, count the number of groups present around each atom. For example 4 groups = sp3, 3 groups = sp2, 2 groups = sp. And for the geometry count the surrounding atoms and lone pairs.
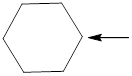
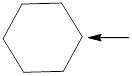
Fig.2
In the given compound (Fig.2), the central atom is carbon. Carbon is surrounding by 4 atoms. So the geometry is tetrahedral. Number of groups present around the nitrogen atom is 4 so the hybridization is sp3.
The geometry of carbon is tetrahedral and the hybridization is sp3.
(c)
Interpretation:
To predict the hybridization and geometry around each indicated atom.
Concept introduction:
Molecular geometry is the three dimensional shape that a molecule in space. It is determine by considering the central atom and the surrounding atom and electron pairs. The shape of the molecule is determined by using Valence Shell Electron Pair Repulsion method. Some of the most common shapes that can be determined by this method are linear, tetrahedral, trigonal planar and pyramidal.
For example.,
Linear (angle = 180o)
Trigonal planar (angle = 120o)
Tetrahedral (angle = 109.5o)
Hybridization is the concept of mixing atomic orbital into new hybrid orbitals suitable for the electron pairing to form chemical bonds and valence bonds in other words mixing of two new orbital having same energy and shape. The orbital is called the hybrid orbital and the process is the hybridization. For example mixing s-orbital and p-orbital to form new hybridization is called sp-hybridization.
Answer to Problem 1.69P
The hybridization and geometry of
![]()
is sp3 and tetrahedral
Explanation of Solution
For the hybridization, count the number of groups present around each atom. For example 4 groups = sp3, 3 groups = sp2, 2 groups = sp. And for the geometry count the surrounding atoms and lone pairs.
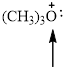
Fig.3
In the given compound (Fig.3), the central atom is oxygen. It is surrounding by 3 atoms and a lone pair. So the geometry is tetrahedral. Number of groups present around the oxygen atom is 4 so the hybridization is sp3.
The geometry is tetrahedral and the hybridization is sp3.
(d)
Interpretation:
To predict the hybridization and geometry around each indicated atom.
Concept introduction:
Molecular geometry is the three dimensional shape that a molecule in space. It is determine by considering the central atom and the surrounding atom and electron pairs. The shape of the molecule is determined by using Valence Shell Electron Pair Repulsion method. Some of the most common shapes that can be determined by this method are linear, tetrahedral, trigonal planar and pyramidal.
For example.,
Linear (angle = 180o)
Trigonal planar (angle = 120o)
Tetrahedral (angle = 109.5o)
Hybridization is the concept of mixing atomic orbital into new hybrid orbitals suitable for the electron pairing to form chemical bonds and valence bonds in other words mixing of two new orbital having same energy and shape. The orbital is called the hybrid orbital and the process is the hybridization. For example mixing s-orbital and p-orbital to form new hybridization is called sp-hybridization.
Answer to Problem 1.69P
The hybridization and geometry of
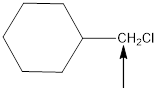
is sp3 and tetrahedral
Explanation of Solution
For the hybridization, count the number of groups present around each atom. For example 4 groups = sp3, 3 groups = sp2, 2 groups = sp. And for the geometry count the surrounding atoms and lone pairs.
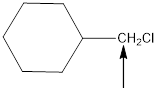
Fig.4
In the given compound(Fig.4), the central atom is carbon. It is surrounding by 4 atoms. So the geometry is tetrahedral. Number of groups present around the carbon atom is 4 so the hybridization is sp3.
The geometry is tetrahedral and the hybridization is sp3.
(e)
Interpretation:
To predict the hybridization and geometry around each indicated atom.
Concept introduction:
Molecular geometry is the three dimensional shape that a molecule in space. It is determine by considering the central atom and the surrounding atom and electron pairs. The shape of the molecule is determined by using Valence Shell Electron Pair Repulsion method. Some of the most common shapes that can be determined by this method are linear, tetrahedral, trigonal planar and pyramidal.
For example.,
Linear (angle = 180o)
Trigonal planar (angle = 120o)
Tetrahedral (angle = 109.5o)
Hybridization is the concept of mixing atomic orbital into new hybrid orbitals suitable for the electron pairing to form chemical bonds and valence bonds in other words mixing of two new orbital having same energy and shape. The orbital is called the hybrid orbital and the process is the hybridization. For example mixing s-orbital and p-orbital to form new hybridization is called sp-hybridization.
Answer to Problem 1.69P
The hybridization and geometry of
![]()
is sp and linear
Explanation of Solution
For the hybridization, count the number of groups present around each atom. For example 4 groups = sp3, 3 groups = sp2, 2 groups = sp. And for the geometry count the surrounding atoms and lone pairs.
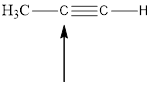
Fig.5
In the given compound (Fig.5), the central atom is carbon. It is surrounding by 2 atoms. So the geometry is linear. Number of groups present around the carbon atom is 2 so the hybridization is sp.
The geometry is linear and the hybridization is sp.
(f)
Interpretation:
To predict the hybridization and geometry around each indicated atom.
Concept introduction:
Molecular geometry is the three dimensional shape that a molecule in space. It is determine by considering the central atom and the surrounding atom and electron pairs. The shape of the molecule is determined by using Valence Shell Electron Pair Repulsion method. Some of the most common shapes that can be determined by this method are linear, tetrahedral, trigonal planar and pyramidal.
For example.,
Linear (angle = 180o)
Trigonal planar (angle = 120o)
Tetrahedral (angle = 109.5o)
Hybridization is the concept of mixing atomic orbital into new hybrid orbitals suitable for the electron pairing to form chemical bonds and valence bonds in other words mixing of two new orbital having same energy and shape. The orbital is called the hybrid orbital and the process is the hybridization. For example mixing s-orbital and p-orbital to form new hybridization is called sp-hybridization.
Answer to Problem 1.69P
The hybridization and geometry of
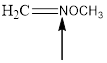
is nitrogen = sp2 and trigonal planar
Explanation of Solution
For the hybridization, count the number of groups present around each atom. For example 4 groups = sp3, 3 groups = sp2, 2 groups = sp. And for the geometry count the surrounding atoms and lone pairs.
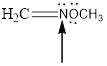
Fig.6
In the given compound (Fig.6), the central atom is nitrogen. Nitrogen is surrounding by 2 atoms and a lone pair. So the geometry is trigonal planar. Number of groups present around the nitrogen atom is 3 so the hybridization is sp2.
The geometry of nitrogen is trigonal planar and the hybridization is sp2.
(g)
Interpretation:
To predict the hybridization and geometry around each indicated atom.
Concept introduction:
Molecular geometry is the three dimensional shape that a molecule in space. It is determine by considering the central atom and the surrounding atom and electron pairs. The shape of the molecule is determined by using Valence Shell Electron Pair Repulsion method. Some of the most common shapes that can be determined by this method are linear, tetrahedral, trigonal planar and pyramidal.
For example.,
Linear (angle = 180o)
Trigonal planar (angle = 120o)
Tetrahedral (angle = 109.5o)
Hybridization is the concept of mixing atomic orbital into new hybrid orbitals suitable for the electron pairing to form chemical bonds and valence bonds in other words mixing of two new orbital having same energy and shape. The orbital is called the hybrid orbital and the process is the hybridization. For example mixing s-orbital and p-orbital to form new hybridization is called sp-hybridization.
Answer to Problem 1.69P
The hybridization and geometry of
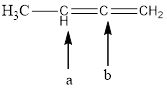
is carbon-a = sp2 and trigonal planar
carbon-b = sp and linear
Explanation of Solution
For the hybridization, count the number of groups present around each atom. For example 4 groups = sp3, 3 groups = sp2, 2 groups = sp. And for the geometry count the surrounding atoms and lone pairs.
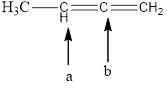
Fig.7
In the given compound (Fig.7), the central atom is carbon. The given structure has two carbons. Carbon-a is surrounding by 3 atoms. So the geometry is trigonal planar. Number of groups present around the carbon atom is 3 so the hybridization is sp2.
Carbon-b is surrounding by 2 atoms. So the geometry is linear. Number of groups present around the carbon atom is 2 so the hybridization is sp.
The geometry of carbon-a is trigonal planar and the hybridization is sp2. The geometry of carbon-b is linear and the hybridization is sp.
Want to see more full solutions like this?
Chapter 1 Solutions
ALEKS 360 CHEMISTRY ACCESS
- Consider the incomplete orbital representation of O2 , below right. a. Identify which lobes are hybrid orbitals (identify the type) and which lobes arep orbitals. b. Use dotted lines to show any bonds. c. Use up or down arrows to show electron occupation of each hybrid orbital or bond.arrow_forwardGive the shape that describes each hybrid orbital set: (a) sp2 (b) sp3d (c) sp (d) sp3d2arrow_forwardPredict the hybridization and geometry around each highlighted atom ?arrow_forward
- CH3+ and CH3− are two highly reactive carbon species. a. What is the predicted hybridization and geometry around each carbon atom? b.Two electrostatic potential plots are drawn for these species. Which ion corresponds to which diagram and why?arrow_forwardWhat is the hybridization and geometry around each labeled atom?arrow_forwarda. what is the hybridization of each non hydrogen atom? b. describe how each (hybrid) atomic orbital is used on each atom. c. how many sigma and pie and non-bonding pairs are there?arrow_forward
- An organic chemist synthesizes the molecule below:(a) Which of the orientations of hybrid orbitals shown below a represent in the molecule? (b) Are there any present that are not shown below? If so, what are they? (c) How many of each type of hybrid orbital are present?arrow_forwardCH3+ and CH3 − are two highly reactive carbon species.a.) What is the predicted hybridization and geometry around each carbonatom?b.) Two electrostatic potential plots are drawn for these species. Whichion corresponds to which diagram and why?arrow_forwardwhich one of the figures below has every sp3 hybridized atom circled?arrow_forward
- identify the hybridization of each carbon in the structurearrow_forwardPlease answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. Determine the hybridization around C2 and nitrogen atoms. What is the expected bond angle around C2 atoms? Identify the type of bonds (σ or π) and the overlapping orbitals that form bonds A and B.arrow_forwardWhat is the hybridization state of each indicated sectionarrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



