
Concept explainers
(a)
Interpretation: The orbitals used to form the indicated bonds are to be predicted.
Concept introduction: The geometry and hybridisation of an atom is determined by the number of group around it. If the number of groups attached to an atom is
Answer to Problem 1.70P
Orbitals used in the formation of given bonds are:
Explanation of Solution
The given compound is,
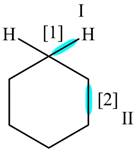
Figure
In bond
Orbitals used in the formation of given bonds are:
(b)
Interpretation: The orbitals used to form the indicated bondsare to be predicted.
Concept introduction: The geometry and hybridisation of an atom is determined by the number of group around it. If the number of groups attached to an atom is
Answer to Problem 1.70P
Orbitals used in the formation of given bonds are:
Explanation of Solution
The given compound is,
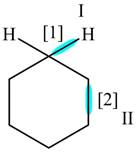
Figure
In bond
Orbitals used in the formation of given bonds are:
(c)
Interpretation: The orbitals used to form the indicated bonds are to be predicted.
Concept introduction: The geometry and hybridisation of an atom is determined by the number of group around it. If the number of groups attached to an atom is
Answer to Problem 1.70P
Orbitals used in the formation of given bonds are:
Explanation of Solution
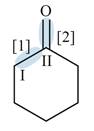
Figure 2
In bond
Orbitals used in the formation of given bonds are:
(d)
Interpretation: The orbitals used to form the indicated bonds are to be predicted.
Concept introduction: The geometry and hybridisation of an atom is determined by the number of group around it. If the number of groups attached to an atom is
Answer to Problem 1.70P
Orbitals used in the formation of given bonds are:
Explanation of Solution
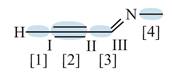
Figure 3
In bond
Orbitals used in the formation of given bonds are:
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry - With Access (Looseleaf) (Custom)
- What orbitals are used to form each highlighted bond? For multiplebonds, indicate the orbitals used in individual bonds ?arrow_forwardAnswer the following questions about erlotinib and terbinafine. Erlotinib,sold under the trade name Tarceva, was introduced in 2004 for thetreatment of lung cancer. Terbinafine is an antifungal medication used totreat ringworm and fungal nail infections. Rank the labeled bonds in terbinafine in order of increasing bondstrength.arrow_forwardchemical compound whose structure is based on C and has many Harrow_forward
- Draw the molecule with bonds and and single bonds if neededarrow_forwardexplain each ans plsarrow_forwardIn HF , neither H nor F holds a full formal charge of +1 or 1 . Organic chemists represent apartial charge using the Greek letter delta () . On the electron density map of the molecule HF above, add a + to one atom and a to the other to indicate which way the bond is polarized.arrow_forward
- A single bond between two carbons with different hybridizations has a small dipole. What is the direction of the dipole in the indicated bonds?arrow_forwardDescribe the orbitals used by each carbon atom in bonding and indicate the approximate bond angles. Drag the appropriate items to their respective bins.arrow_forwardPlease provide the hybridization for each carbon.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


