
Concept explainers
Draw a Lewis structure for each ion.
a.
(a)
Interpretation: The Lewis structure for
Concept Introduction: Lewis structures are electron dot representations for molecules. These structures show the bonding between the atoms or molecules and the lone pairs of electrons. The arrangement of valence electrons among the atoms in a molecule is represented by its Lewis structure.
Answer to Problem 1.7P
The Lewis structure for
Explanation of Solution
In the given molecular formula,
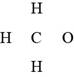
Figure 1
The total valence electrons in
The bonds and lone pairs are added to form
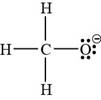
Figure 2
The Lewis structure for
(b)
Interpretation: The Lewis structure for
Concept Introduction: Lewis structures are electron dot representations for molecules. These structures show the bonding between the atoms or molecules and the lone pairs of electrons. The arrangement of valence electrons among the atoms in a molecule is represented by its Lewis structure.
Answer to Problem 1.7P
The Lewis structure of
Explanation of Solution
In the given molecular formula,
![]()
Figure 3
The total valence electrons in
The bonds and lone pairs are added to form
![]()
Figure 4
The Lewis structure for
![]()
Figure 5
The Lewis structure of
(c)
Interpretation: The Lewis structure for
Concept Introduction: Lewis structures are electron dot representations for molecules. These structures show the bonding between the atoms or molecules and the lone pairs of electrons. The arrangement of valence electrons among the atoms in a molecule is represented by its Lewis structure.
Answer to Problem 1.7P
The Lewis structure of
Explanation of Solution
In the given molecular formula,
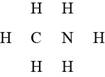
Figure 6
The total valence electrons in
The bonds and lone pairs are added to form
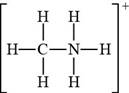
Figure 7
The Lewis structure of
(d)
Interpretation: The Lewis structure for
Concept Introduction: Lewis structures are electron dot representations for molecules. These structures show the bonding between the atoms or molecules and the lone pairs of electrons. These structures can be drawn for any covalently bonded molecules.
Answer to Problem 1.7P
The Lewis structure of
Explanation of Solution
The given molecular formula,
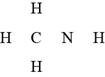
Figure 8
The total valence electrons in
The bonds and lone pairs are added to form
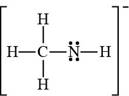
Figure 9
The Lewis structure of
Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEMISTRY-MOLYMOD PACKAGE
Additional Science Textbook Solutions
Chemistry In Context
Chemistry: Matter and Change
Chemistry (7th Edition)
Living By Chemistry: First Edition Textbook
Organic Chemistry As a Second Language: Second Semester Topics
- How many lone pairs are there on the central C atom in the Lewis structure for CH4 ? How many lone pairs are there on the central C atom in the Lewis structure for CH4 ? 1 3 4 0 2arrow_forward1) Discuss the advantages and disadvantages of using the molecular models (ball and stick) as learning aids. Recommend an alternative to using this method for modeling molecules. 2) Build C6H6 as a ring. What do you notice about the placement of the bonds? This is an example of resonance. What is a resonant Lewis structure? 3) Explain how a diminished and expanded octet can be formed.arrow_forwardI2(g) + Br2(g) ---> 2IBr(g) Draw Lewis structures for all reactant and product molecules.arrow_forward
- 1. What is the formal charge on each atom in CH3Cl and the Lewis structure for it? 2. What is the formal charge on each atom in PH4 nd the Lewis structure for it? 3. What is the formal charge on each atom in HNO3 and the Lewis structure for it?arrow_forwardWrite the Lewis structure for each molecule. SiH4arrow_forwardWhich two species have the same number of lone electron pairs in their Lewis structures?(a) H2O and H3O+(b) NH3 and H3O+(c) NH3 and CH4(d) NH3 and NH4+arrow_forward
- CH3OH(g) + HI(g) CH3I(g) + H2O(g) draw Lewis structures for all reactant and product molecules.arrow_forwardExperimental evidence indicates the existence of HC3N molecules in interstellar clouds. Write a plausible Lewis structure for this molecule.arrow_forwardWhich molecule has the most polar bond: N2, BrF, or ClF? Use an arrow to show the direction of polarity in each bond.arrow_forward
- Consider the following compounds: CO2, SO2, KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, TF5, and SCl6. These 12 compounds are all examples of different molecular structures. Draw the Lewis structures for each and predict the molecular structures. Predict the bond angles and the polarity of each. (A polar molecule has a net dipole moment, while a nonpolar molecule does not.) See Exercises 25 and 26 for the molecular structures based on the trigonal bipyramid and the octahedral geometries.arrow_forwardConsider the following compounds: CO2, SO2, KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, IF5, and SCl6. These 12 compounds arc all examples of different molecular structures. Draw the Lewis structures for each and predict the molecular structure. Predict the bond angles and the polarity of each. (A polar molecule has a net dipole moment, while a nonpolar molecule docs not.) See Exercises 115 and 116 for the molecular structures based on the trigonal bipyramid and the octahedral geometries.arrow_forwardDraw Lewis structures for the following species. (The skeleton is indicated by the way the molecule is written.) (a) Cl2CO (b) H3C—CN (c) H2C—CH2arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





