
(a)
Interpretation: The constitution isomers of the compounds have to be found by using their molecular formula.
Concept introduction: The arrangement of atoms that are bonded together determines its constitution and molecular formula of that particular compound.
This concept is referred as structural isomers or in more modern term constitutional isomers.
Each atom has a typical valency or valence which is defined as the ability of an atom to form a
Nitrogen and oxygen atoms are trivalent and divalent respectively. Hydrogen and halogens are monovalent in nature.
To find: All the constitutional isomers for the compound C4H10
(a)
Answer to Problem 34PP
The constitution isomers for the compound C4H10 are
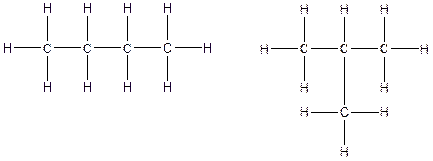 (a).
(a).
Explanation of Solution
Carbon (C), hydrogen (H) valency in C4H10
Carbon is tetravalent whereas hydrogen is monovalent respectively. In the given compound, there are four carbon atoms and ten hydrogen atoms. Carbon atom is able to form four chemical bonds with other atoms. Similarly, each hydrogen atoms are bonded by a chemical bond.
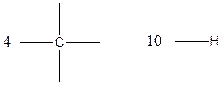
The arrangement of atoms for the constituational isomers C4H10
There are two possible ways to draw the main chains of atoms. Carbon has the highest valency in the given compound. In the first possible way, all the four carbon atoms occupy the centered position by the formation of three carbon-carbon single bonds in a linear manner. In the second way, three carbon atoms are arranged as the main chain with one carbon atom in a branched manner.
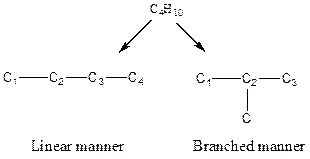
Hydrogen atoms occupy the remaining peripheral positions.
Therefore, all the possible constitutional isomers of the compound C4H10 are
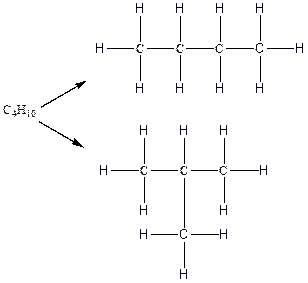
(b)
Interpretation: The constitution isomers of the compounds have to be found by using their molecular formula.
Concept introduction: The arrangement of atoms that are bonded together determines its constitution and molecular formula of that particular compound.
This concept is referred as structural isomers or in more modern term constitutional isomers.
Each atom has a typical valency or valence which is defined as the ability of an atom to form a chemical bond with other atoms. For example, carbon has four valence or tetravalent that means carbon has the capacity to form four bonds with other elements or other atoms.
Nitrogen and oxygen atoms are trivalent and divalent respectively. Hydrogen and halogens are monovalent in nature.
To find: All the constitutional isomers for the compound C5H12
(b)
Answer to Problem 34PP
The constitution isomers for the compound C5H12 are
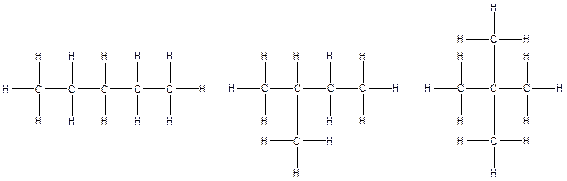
(b).
Explanation of Solution
To find Carbon (C), hydrogen (H) valency in C5H12
Carbon is tetravalent whereas hydrogen is monovalent respectively. In the given compound, there are five carbon atoms and twelve hydrogen atoms. Carbon atom is able to form four chemical bonds with other atoms. Similarly, each hydrogen atoms are bonded by a chemical bond.
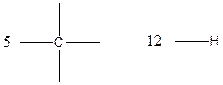
The arrangement of atoms for the constituational isomers C5H12
There are three possible ways to draw the main chains of atoms. Carbon has the highest valency in the given compound. In the first possible way, all the five carbon atoms occupy the centered position by the formation of four carbon-carbon single bonds in a linear manner. In the second way, four carbon atoms are arranged as the main chain with one carbon atom in a branched manner. In the third way, three carbon atoms are arranged as the main chain with two carbon atoms in a branched manner.
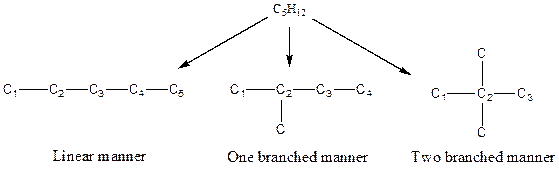
Hydrogen atoms occupy the remaining peripheral positions.
Therefore, all the possible constitutional isomers of the compound C5H12 are
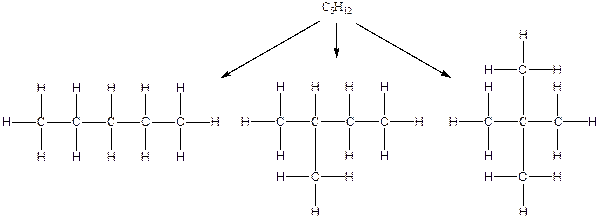
(c)
Interpretation: The constitution isomers of the compounds have to be found by using their molecular formula.
Concept introduction: The arrangement of atoms that are bonded together determines its constitution and molecular formula of that particular compound.
This concept is referred as structural isomers or in more modern term constitutional isomers.
Each atom has a typical valency or valence which is defined as the ability of an atom to form a chemical bond with other atoms. For example, carbon has four valence or tetravalent that means carbon has the capacity to form four bonds with other elements or other atoms.
Nitrogen and oxygen atoms are trivalent and divalent respectively. Hydrogen and halogens are monovalent in nature.
To find: All the constitutional isomers for the compound C6H14
(c)
Answer to Problem 34PP
The constitution isomers for the compound C6H14 are
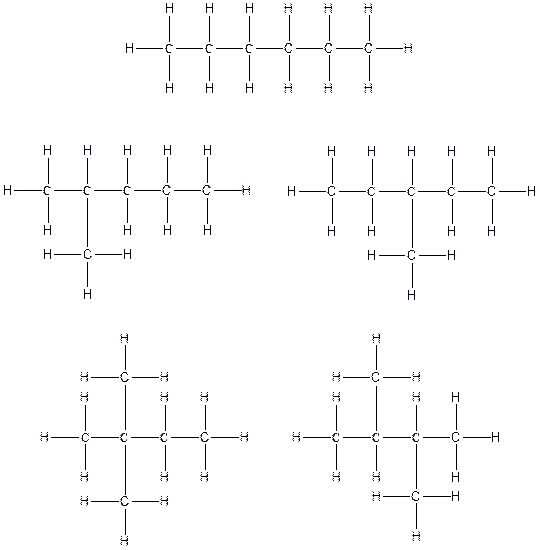 (c).
(c).
Explanation of Solution
Carbon (C), hydrogen (H) valency in C6H14
Carbon is tetravalent whereas hydrogen is monovalent respectively. In the given compound, there are six carbon atoms and fourteen hydrogen atoms. Carbon atom is able to form four chemical bonds with other atoms. Similarly, each hydrogen atoms are bonded by a chemical bond.
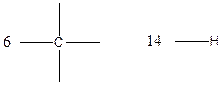
The arrangement of atoms for the constituational isomers C6H14
There are three possible ways to draw the main chains of atoms. Carbon has the highest valency in the given compound. In the first possible way, all the six carbon atoms occupy the centered position by the formation of five carbon-carbon single bonds in a linear manner. So, one linear structure is possible in this way. In the second way, five carbon atoms are arranged as the main chain with one carbon atom in a branched manner. So, two single carbon branched structures are possible in this way. In the third way, four carbon atoms are arranged as the main chain with two carbon atoms in a branched manner. So, two branched structures which form due to two carbons are possible in this way.
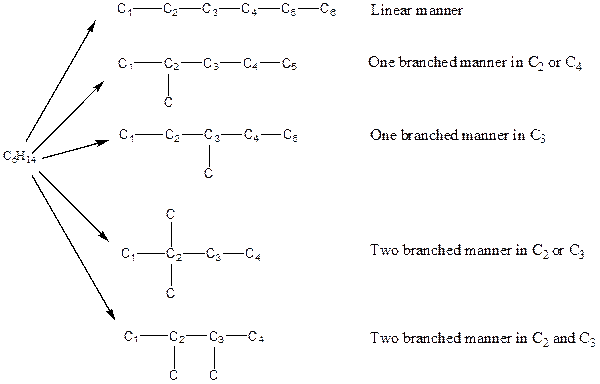
Hydrogen atoms occupy the remaining peripheral positions.
Therefore, all the possible constitutional isomers of the compound C6H14 are
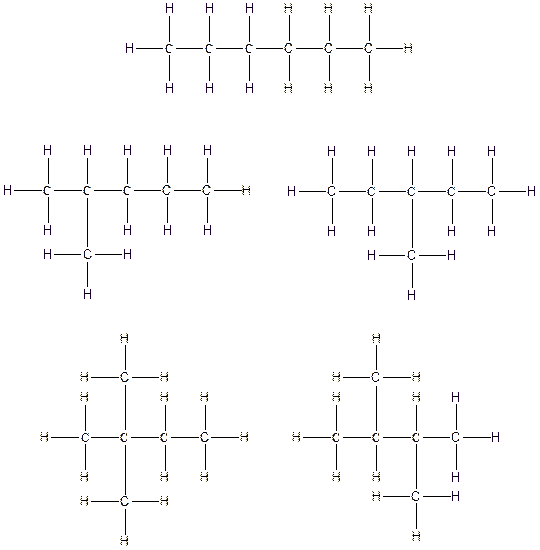
(d)
Interpretation: The constitution isomers of the compounds have to be found by using their molecular formula.
Concept introduction: The arrangement of atoms that are bonded together determines its constitution and molecular formula of that particular compound.
This concept is referred as structural isomers or in more modern term constitutional isomers.
Each atom has a typical valency or valence which is defined as the ability of an atom to form a chemical bond with other atoms. For example, carbon has four valence or tetravalent that means carbon has the capacity to form four bonds with other elements or other atoms.
Nitrogen and oxygen atoms are trivalent and divalent respectively. Hydrogen and halogens are monovalent in nature.
To find: The constitutional isomer for the compound C2H5Cl
(d)
Answer to Problem 34PP
The constitution isomer for the compound C2H5Cl is
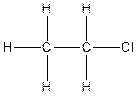 (d).
(d).
Explanation of Solution
Carbon (C), hydrogen (H) and chlorine (Cl) atoms valency in C2H5Cl
Carbon is tetravalent whereas both chlorine and hydrogen atoms are monovalent. In the given compound, there are two carbon atoms, one chlorine atom and five hydrogen atoms. Carbon atom is able to form four chemical bonds with other atoms. Similarly, chlorine and each hydrogen atoms are bonded by a single chemical bond.
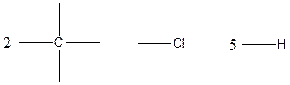
The arrangement of atoms for the constituational isomer C2H5Cl
Carbon atom has the highest valency in the given compound. So, it occupies the centered position by the formation of a single carbon-carbon bond. In the case of hydrogen and chlorine atoms, they occupy the peripheral positions.
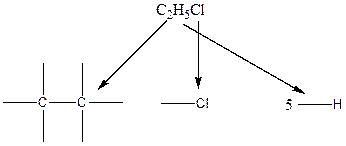
The chlorine atom can attach in any position of the six available positions of two carbon atoms. The resulting six structures are identical.
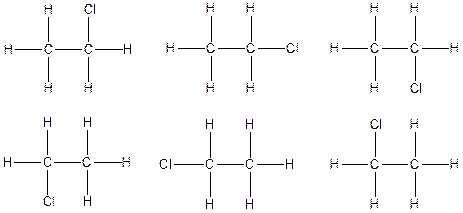
Therefore, the arrangenment of atoms present in the constitutional isomer C2H5Cl is as follows:

(e)
Interpretation: The constitution isomers of the compounds have to be found by using their molecular formula.
Concept introduction: The arrangement of atoms that are bonded together determines its constitution and molecular formula of that particular compound.
This concept is referred as structural isomers or in more modern term constitutional isomers.
Each atom has a typical valency or valence which is defined as the ability of an atom to form a chemical bond with other atoms. For example, carbon has four valence or tetravalent that means carbon has the capacity to form four bonds with other elements or other atoms.
Nitrogen and oxygen atoms are trivalent and divalent respectively. Hydrogen and halogens are monovalent in nature.
To find: The constitutional isomers for the compound C2H4Cl2
(e)
Answer to Problem 34PP
The constitution isomers for the compound C2H4Cl2 are
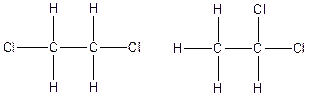 (e).
(e).
Explanation of Solution
Carbon (C), hydrogen (H) and chlorine (Cl) atoms valency in C2H4Cl2
Carbon is tetravalent whereas both chlorine and hydrogen atoms are monovalent. In the given compound, there are two carbon atoms, two chlorine atoms and four hydrogen atoms. Carbon atom is able to form four chemical bonds with other atoms. Similarly, chlorine and each hydrogen atoms are bonded by a single chemical bond.
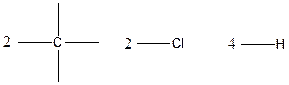
The arrangement of atoms for the constituational isomers C2H4Cl2
Carbon atom has the highest valency in the given compound. So, it occupies the centered position by the formation of a single carbon-carbon bond.
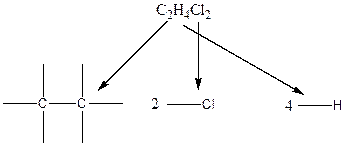
In the case of hydrogen and chlorine atoms, they occupy the peripheral positions. Chlorine atoms can attach to carbon atoms in two different ways. In the first way, each chlorine atom is attached to each carbon atom. In the second way, both the chlorine atoms are connected to only one carbon atom.
Therefore, the arrangenment of atoms present in the constitutional isomers C2H4Cl2 is as follows:
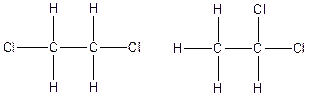
(f)
Interpretation: The constitution isomers of the compounds have to be found by using their molecular formula.
Concept introduction: The arrangement of atoms that are bonded together determines its constitution and molecular formula of that particular compound.
This concept is referred as structural isomers or in more modern term constitutional isomers.
Each atom has a typical valency or valence which is defined as the ability of an atom to form a chemical bond with other atoms. For example, carbon has four valence or tetravalent that means carbon has the capacity to form four bonds with other elements or other atoms.
Nitrogen and oxygen atoms are trivalent and divalent respectively. Hydrogen and halogens are monovalent in nature.
To find: The constitutional isomers for the compound C2H3Cl3
(f)
Answer to Problem 34PP
The constitution isomers for the compound C2H3Cl3 are
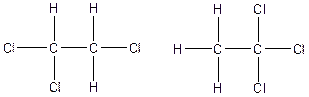 (f).
(f).
Explanation of Solution
Carbon (C), hydrogen (H) and chlorine (Cl) atoms valency in C2H3Cl3
Carbon is tetravalent whereas both chlorine and hydrogen atoms are monovalent. In the given compound, there are two carbon atoms, three chlorine atoms and three hydrogen atoms. Carbon atom is able to form four chemical bonds with other atoms. Similarly, chlorine and each hydrogen atoms are bonded by a single chemical bond.
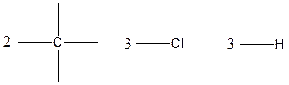
The arrangement of atoms for the constituational isomers C2H3Cl3
Carbon atom has the highest valency in the given compound. So, it occupies the centered position by the formation of a single carbon-carbon bond.
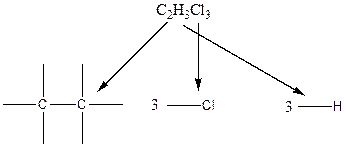
In the case of hydrogen and chlorine atoms, they occupy the peripheral positions. Chlorine atoms can attach to carbon atoms in two different ways. In the first way, two chlorine atoms are attached to one carbon atom and one chlorine atom is attached to another carbon atom. In the second way, all the chlorine atoms are connected to only one carbon atom.
Therefore, the arrangenment of atoms present in the constitutional isomers C2H3Cl3 is as follows:
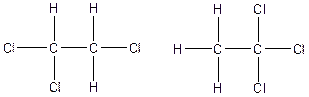
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





