
(a)
To determine: Whether the entropy increases or decreases in the given process.
Given process:
Introduction: The entropy is based on the concept that is in the isolated system, where everything in nature tends to move from order to disorder. It is a state of randomness in matter.
(b)
To determine: Whether the entropy increases or decreases in the given process.
Given process:
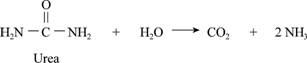
Fig.1
Introduction: The entropy is based on the concept that in an isolated system everything in nature tends to move from order to disorder. It is a state of randomness in matter.
(c)
To determine: Whether the entropy increases or decreases in the given process.
Given process:

Fig. 2
Introduction: The entropy is based on the concept that is in the isolated system, where everything in nature tends to move from order to disorder. It is a state of randomness in matter.
(d)
To determine: Whether the entropy increases or decreases in the given process.
Given process:
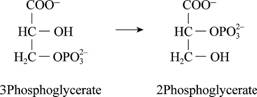
Fig. 3
Introduction: The entropy is based on the concept that is in the isolated system, where everything in nature tends to move from order to disorder. It is a state of randomness in matter.
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video

Chapter 1 Solutions
Fundamentals of Biochemistry: Life at the Molecular Level
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





