
(a)
Interpretation:
The structure of sodium isopropoxide is to be predicted.
Concept introduction:
The systematic naming of organic compound is given by
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 10.1P
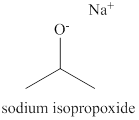
The structure of sodium isopropoxide is shown below.
Explanation of Solution
The name of the compound sodium isopropoxide indicates that the compound is organic salt. The cation of the sodium isopropoxide is sodium ion and anion of this compound is isopropoxide ion. The isopropoxide ion has isopropyl group attracted to oxygen atom. The charge on isopropoxide ion is

Figure 1
The structure of sodium isopropoxide is shown in Figure 1.
(b)
Interpretation:
The structure of potassium tert-butoxide is to be predicted.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 10.1P
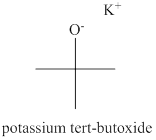
The structure of potassium tert-butoxide is shown below.
Explanation of Solution
The name of the compound potassium tert-butoxide indicates that the compound is organic salt. The cation of the potassium tert-butoxide is potassium ion and anion of this compound is tert-butoxide ion. The tert-butoxide ion has tert-butyl group attracted to oxygen atom. The charge on tert-butoxide ion is
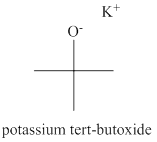
Figure 2
The structure of potassium tert-butoxide is shown in Figure 2.
(c)
Interpretation:
The structure of
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 10.1P
The structure of
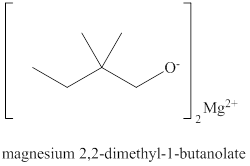
Explanation of Solution
The name of the compound

Figure 3
The structure of
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry
- An organic chemist was subjected to sodium fusion and the resulting lassaigne's extract boiled with FeSO4 and acidified with concentrated H2SO4, yielding a prussian blue color. Give the structure of one of the organic compounds which fits the description.arrow_forwardBriefly discuss the following topics and include appropriate examples where necessary. Extremely thermophilic sulphur metabolizers.arrow_forwardA neutral compound A (C7H5N) is reduced to compound B (C7H9N) which is insoluble in water but dissolves in dilute hydrochloric acid. Compound B reacts with cold nitrous acid to produce compound C (C7H8O) with the evolution of a colourless gas. Oxidation of compound C produces compound D (C7H6O2). Compound D is also obtained when compound A is boiled with dilute sulphuric acid. Compound D reacts with phosphorus (V) chloride to produce compound E (C7H5ClO). Compound E reacts with compound C to produce compound F (C14H12O2) which is an ester. (a) Draw the structural formula of all the compounds A-F. (b) Write a complete equation for the formation of compound B. (c) Write a complete equation for the formation of compound C. (d) Write a complete equation for the reactions that occur for compound D when compound A is boiled with dilute sulphuric acid. (e) Write a complete equation for the formation of compound E. (f) Write a complete equation for the formation of compound F. (g) Give…arrow_forward
- Deduce the structure of this compound.arrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Questions: (i) Give the name and suggest the colour of the precipitate B. Hence explain why it is necessary to recrystallize B several times. (ii) Why was it necessary to obtain a constant melting point for B?arrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. 1g of phenylamine yielded 1.2g of compound A. Calculate the percentage yield of the reaction.arrow_forward
- The following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Why was it necessary to obtain a constant melting point for B?arrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Give the structure of the product formed when an acidified solution of compound A is reacted with (i)Naphthalen-2-ol (2-naphthol) (ii) Sodium cyanidearrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Question: Answer both (i) and (ii) below (i) Give the structure of the product formed when an acidified solution of compound A is reacted with Naphthalen-2-ol (2-naphthol) and Sodium cyanide separately (ii) 1g of phenylamine yielded 1.2g of compound A. Calculate the percentage yield of the reaction.arrow_forward
- The following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Write the equation for the reaction between compound A and phenol Give the name and suggest the colour of the precipitate B. Why is it necessary to recrystallize B several times?arrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. (a) What is the general name given to the reaction between phenylamine and sodium nitrite and explain why it's necessary to carry out the reaction at low temperatures. (b) Write the equation for the reaction between compound A and phenolarrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. What is the general name given to the reaction between phenylamine and sodium nitrite and explain why it's necessary to carry out the reaction at low temperatures? Write the equation for the reaction between compound A and phenol Write the equation for the reaction between phenylamine and sodium nitrite.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





