
Concept explainers
a.
To draw:
The structure of alanine and mark an asterisk (*) next to any chiral carbon centers.
Introduction:
Amino acids contain a protonated
a.
Explanation of Solution
Alanine is a non essential amino acid. The three letter abbreviation of alanine is “Ala” and the one letter code is “A”. The side chain of alanine contains hydrocarbon as functional group.
Pictorial representation:
The structure of amino acid Alanine.
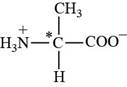
Fig 1: The structure of amino acid Alanine (Ala, A).
b.
To determine:
The structure of lysine and mark an asterisk (*) next to any chiral carbon centers
Introduction:
Amino acids contain a protonated amine and a carboxylic acid in the form of carboxylate ion. These two functional groups are bonded to a central carbon atom called the alpha-carbon. The protonated amine bonded to this carbon is called alpha-amino group and the carboxylate ion as the alpha carboxylate group. The alpha-carbon is also bonded to a hydrogen atom and a large side chain designated as R, which gives unique identification and characteristics to each amino acids.
b.
Explanation of Solution
Lysine is an essential amino acid. The three letter abbreviation of lysine is “Lys” and the one letter code is “K”. The side chain of lysine contains protonated amine as functional group.
Pictorial representation: The structure of amino acid Lysine.
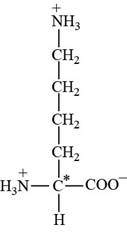
Fig 2: The structure of amino acid Lysine (Lys, K).
c.
To determine:
The structure of tryptophan and mark an asterisk (*) next to any chiral carbon centers
Introduction:
Amino acids contain a protonated amine and a carboxylic acid in the form of carboxylate ion. These two functional groups are bonded to a central carbon atom called the alpha-carbon. The protonated amine bonded to this carbon is called alpha-amino group and the carboxylate ion as the alpha carboxylate group. The alpha-carbon is also bonded to a hydrogen atom and a large side chain designated as R, which gives unique identification and characteristics to each amino acids.
c.
Explanation of Solution
Tryptophan is an essential amino acid. The three letter abbreviation of tryptophan is “Trp” and the one letter code is “W”. The side chain of tryptophan contains
Pictorial representation:
The structure of amino acid Tryptophan.
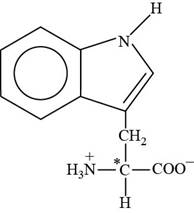
Fig 3: The structure of amino acid Tryptophan (Trp, W).
d.
To determine:
The structure of aspartate and mark an asterisk (*) next to any chiral carbon centers
Introduction:
Amino acids contain a protonated amine and a carboxylic acid in the form of carboxylate ion. These two functional groups are bonded to a central carbon atom called the alpha-carbon. The protonated amine bonded to this carbon is called alpha-amino group and the carboxylate ion as the alpha carboxylate group. The alpha-carbon is also bonded to a hydrogen atom and a large side chain designated as R, which gives unique identification and characteristics to each amino acids.
d.
Explanation of Solution
Aspartate is a non essential amino acid. The three letter abbreviation of aspartate is “Asp” and the one letter code is “D”. The side chain of aspartate contains carboxylate as functional group.
Pictorial representation:
The structure of amino acid Aspartate.
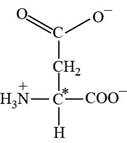
Fig 4: The structure of amino acid Aspartate (Asp, D)
Want to see more full solutions like this?
Chapter 10 Solutions
General, Organic, and Biological Chemistry, Books a la Carte Edition (3rd Edition)

 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





