
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.57P
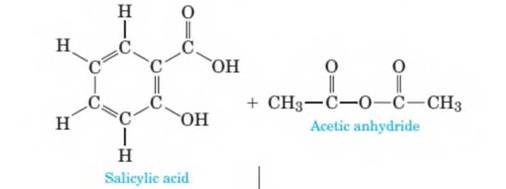
Aspirin is prepared by the reaction of salicylic- acid with acetic anhydride as shown in the following equation. The stoichiometry of the reaction is given in the equation. Acetic acid is a by-product of the reaction and must be separated and removed so that aspirin can then be sold as a pure product. How many grams of aspirin can be prepared from 120 grams of salicylic acid? Assume that there is an excess of acetic anhydride. (Chapter 4)
Acetylsalieylic acid
(Aspirin)
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Write the chemical equation for the reaction of propanoic acid with 1-butanol (an alcohol).
The formula of 1-butanol is CH-CH-CH2-CH2-OH.
Which is formula of the this rule of reaction?
“When methyl propanoate is hydrolyzed, formic acid and 1-propanol are formed.” If this statement is true, write a balanced chemical reaction for it. If it is false, explain why.
Which statement is true?
Statement 1: In the synthesis of aspirin, sulfuric acid serves as the catalyst to speed up the reaction and is consumed in the course of the reaction.
Statement 2: In the synthesis of aspirin, salicylic acid and acetic anhydride are the starting materials, acetylsalicylic acid is the final product and acetic acid is the by-product.
Chapter 10 Solutions
Introduction to General, Organic and Biochemistry
Ch. 10.3 - Prob. 10.1PCh. 10.4 - Prob. 10.2PCh. 10.4 - Prob. 10.3PCh. 10.4 - Prob. 10.4PCh. 10.4 - Prob. 10.5PCh. 10.4 - Prob. 10.6PCh. 10 - Prob. 10.7PCh. 10 - Prob. 10.8PCh. 10 - 10-9 Is there any difference between vanillin made...Ch. 10 - Prob. 10.10P
Ch. 10 - 10-11 What important experiment did Wohler carry...Ch. 10 - Prob. 10.12PCh. 10 - Prob. 10.13PCh. 10 - Prob. 10.14PCh. 10 - 10-15 How many electrons are in the valence shell...Ch. 10 - 10-16 What is the relationship between the number...Ch. 10 - Prob. 10.17PCh. 10 - Prob. 10.18PCh. 10 - 10-19 Write Lewis structures for these ions. (a)...Ch. 10 - 10-20 Why are the following molecular formulas...Ch. 10 - 10-21 Explain how to use the valence-shell...Ch. 10 - 10-22 Suppose you forget to take into account the...Ch. 10 - Suppose you forget to take into account the...Ch. 10 - 10-24 Use the VSEPR model to predict the bond...Ch. 10 - Prob. 10.25PCh. 10 - Prob. 10.26PCh. 10 - 10-27 What is meant by the term functional group?Ch. 10 - 10-28 List three reasons why functional groups are...Ch. 10 - Prob. 10.29PCh. 10 - Prob. 10.30PCh. 10 - Prob. 10.31PCh. 10 - 10-32 Draw a structural formula for the one...Ch. 10 - 10-33 What is the meaning of the term tertiary (...Ch. 10 - Prob. 10.34PCh. 10 - Draw structural formulas for each of the...Ch. 10 - 10-36 Draw structural formulas for the six ketones...Ch. 10 - 10-37 Draw structural formulas for the eight...Ch. 10 - Prob. 10.38PCh. 10 - 10-39 (Chemical Connections 10A) How was Taxol...Ch. 10 - Prob. 10.40PCh. 10 - Prob. 10.41PCh. 10 - Silicon is immediately below carbon in Group 4A of...Ch. 10 - 10-43 Phosphorus is immediately below nitrogen in...Ch. 10 - Draw the structure for a compound with the...Ch. 10 - 10-45 Draw structural formulas for the eight...Ch. 10 - Prob. 10.46PCh. 10 - 10-47 Which of these covalent bonds are polar, and...Ch. 10 - Of the bonds in Problem 10-47, which is the most...Ch. 10 - Prob. 10.49PCh. 10 - Prob. 10.50PCh. 10 - Following is a structural formula for naphthalene....Ch. 10 - Prob. 10.52PCh. 10 - Prob. 10.53PCh. 10 - Urea, (NH.,)2CO, is used in plastics and in fertil...Ch. 10 - Prob. 10.55PCh. 10 - Prob. 10.56PCh. 10 - Aspirin is prepared by the reaction of salicylic-...Ch. 10 - Following is the structural formula of acetamide....Ch. 10 - Prob. 10.59P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the roles of methyl salicylate and sulfuric acid in the synthesis of salicylic acid?arrow_forward(a) When the metallic element sodium combines with the nonmetallic element bromine, Br2(l), how can you determine the chemical formula of the product? How do you know whether the product is a solid, liquid, or gas at room temperature? Write the balanced chemical equation for the reaction. (b) When a hydrocarbon burns in air, what reactant besides the hydrocarbon is involved in the reaction? What products are formed? Write a balanced chemical equation for the combustion of benzene C6H6(l), in air.arrow_forwardThe combustion of methane is represented by the equation:CH4 + 2O2 → CO2 + 2H2Oa) In the above reaction what compound is oxidized?b) Give another example of a hydrocarbon combustion reaction and write the equation.arrow_forward
- Explain by equations how organophosphate pesticides can harm humans.arrow_forwardConsider this statement: “When methyl propanoate is hydrolyzed, formic acid and 1-propanol are formed.” If this statement is true, write a balanced chemical reaction for it. If it is false, explain why.arrow_forwardWrite the structure of the polymer, tetrafluoroethylenearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License