
Concept explainers
Give the structure corresponding to each IUPAC name.
- a. 3-ethylhexane
- b. 3-ethyl-3-methyloctane
- c. 2,3,4,5-tetramethyldecane
- d. cyclononane
- e. 1,1,3-trimethylcyclohexane
a.
Interpretation:
The structure of given IUPAC name 3-ethylhexane has to be drawn.
Concept Introduction:
IUPAC Nomenclature:
The system which is used to name an organic compound is known as IUPAC Nomenclature. IUPAC are known as International Union of Pure and Applied Chemistry.
There are some rules followed for writing IUPAC Nomenclature for alkane compound is given below,
- The longest chain in the compound is known as parent name which represent the number of carbon atom present in the continuous carbon chain. The root name for alkane compound is –ane. The single bonds are formed in alkane compounds.
- The suffix group denotes the functional group present in a molecule. The prefix group indicates the identity, location and number of substituents attached to the carbon compound.
- Then name and number the substituents present in compounds. Then use prefix di-to represent two groups, tri- refers to three groups and so on. Then numbers has to be separated by using commas and the letters from numbers has to be separated by using dashes. Then numbers has to be separated by using commas and the letters from numbers has to be separated by using dashes.
There are some rules followed for writing IUPAC Nomenclature for cycloalkane compounds:
- First identify the number of carbon atoms present in the ring. The numbers of carbon atoms present in ring is used as the parent name. Add suffix name -ane and prefix name cyclo- to the parent name of cycloalkane compound.
- The suffix group denotes the functional group present in a molecule. Then number the substituents present in compound. If there is a single substituent no need to mention number. If the ring has more than one substituent, then start numbering the ring with lowest number to the substituents in alphabetical order. Hence combine the parts to form IUPAC name.
Explanation of Solution
The structure of given IUPAC name 3-ethylhexane has to be drawn. The longest continuous chain has six carbon atoms and so the parent name is hex-. The root name of alkane compounds are –ane has to be added as suffix name to the parent name. Hence the parent name is given as hexane.
The carbon skeleton for hexane is given below,
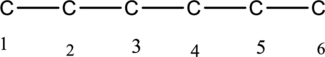
Then number and name the substituent in alphabetical order. The substituent ethyl group
The substituent linked to carbon chain is given below,
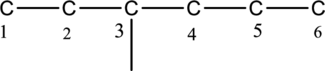
The structure of given IUPAC name 3-ethylhexane is drawn below,
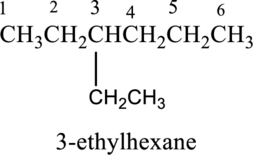
b.
Interpretation
The structure of given IUPAC name 3-ethyl-3-methyloctane has to be drawn.
Concept Introduction:
Refer part a.
Explanation of Solution
The structure of given IUPAC name 3-ethyl-3-methyloctane has to be drawn. The longest continuous chain has eight carbon atoms and so the parent name is oct-. The root name of alkane compounds are –ane has to be added as suffix name to the parent name. Hence the parent name is given as octane.
The carbon skeleton for octane is given below,
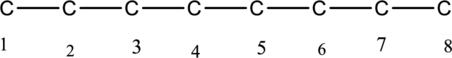
Then number and name the substituents in alphabetical order. The substituent ethyl group
The substituents linked to carbon chain is drawn below,
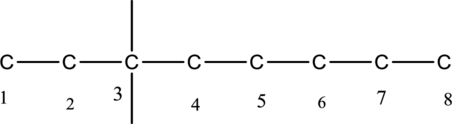
The structure of given IUPAC name 3-ethyl-3-methyloctane is drawn below,
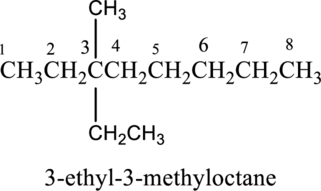
c.
Interpretation
The structure of given IUPAC name 2,3,4,5, -tetramethyldecane has to be drawn.
Concept Introduction:
Refer part a.
Explanation of Solution
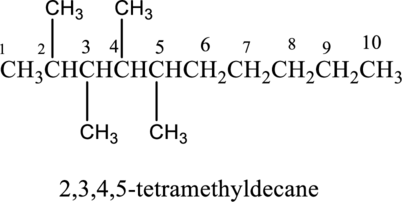
The structure of given IUPAC name 2,3,4,5-tetramethyldecane has to be drawn. The longest continuous chain has ten carbon atoms and so the parent name is dec-. The root name of alkane compounds are –ane has to be added as suffix name to the parent name. Hence the parent name is given as decane.
The carbon skeleton for decane is drawn below,
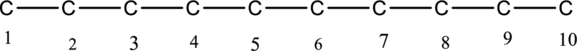
Then number and name the substituents in alphabetical order. The substituent methyl groups are bonded to second, third, fourth and fifth carbon position. Then prefix tetra is used to refer the four methyl groups. Then numbers has to be separated by using commas and the letters from numbers has to be separated by using dashes. Hence the structure of given IUPAC name 2,3,4,5-tetramethyldecane.
The substituent is attached to carbon chain is drawn below,
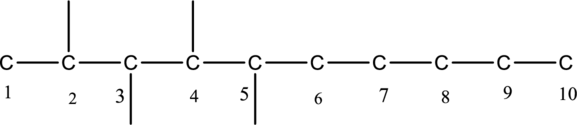
The structure of given compound 2,3,4,5, - tetramethyldecane is given below,
d.
Interpretation
The structure of given IUPAC name cyclononane has to be drawn.
Concept Introduction:
Refer part a.
Explanation of Solution
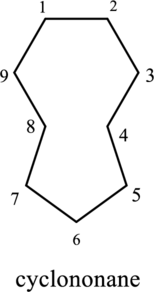
The cyclic structure has nine carbon atoms and it is given as parent name. Hence the name can be given as cyclononane. In this compound there is no substituent group. There is no need to mention the numbering for single substituent.
The structure of given compound cyclononane is drawn below,
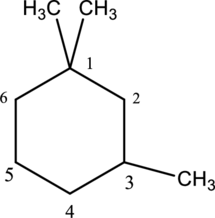
e.
Interpretation
The structure of given IUPAC name 1,1,3-trimethylcyclohexane has to be drawn.
Concept Introduction:
Refer part a.
Explanation of Solution
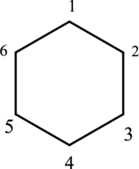
The given IUPAC name 1,1,3-trimethylcyclohexane, where hexane denotes the six number of carbon atoms is present in the cyclic structure. Hence the parent name is given as hexane.
The structure for cyclohexane is a cyclic structure and is drawn below,
The three methyl substituents are bonded to first and third carbon. Then number and name the substituents in alphabetical order. The prefix tri- is used to represent three methyl groups. Hence the structure of given IUPAC name 3-ethylhexane.
The substituent attached to the cyclic structure is drawn below,
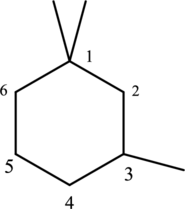
The structure of given compound 1,1,3 –trimethylcyclohexane is drawn below,
Want to see more full solutions like this?
Chapter 10 Solutions
Principles of General, Organic, Biological Chemistry
- 1. 1-Ethoxy-2-methylpropane A. Acid anhydride B. Acid chloride C. Ether D. None of the choices 2. C8H16 A. Alkane B. Alkene C. Cycloalkane D. Two of the choices 3. How many CH2 is present in this compound? (Please refer to the timage attached.) A. 6 B. 5 C. 4 D. 3arrow_forwardDraw the structure that corresponds with each name.(a) 3-ethyloctane (b) 4-isopropyldecane (c) sec-butylcycloheptane(d) 2,3-dimethyl-4-propylnonane (e) 2,2,4,4-tetramethylhexane (f) trans-1,3-diethylcyclopentane(g) cis-1-ethyl-4-methylcyclohexane (h) isobutylcyclopentane (i) tert-butylcyclohexane(j) pentylcyclohexane (k) cyclobutylcyclohexane (l) cis-1-bromo-3-chlorocyclohexanearrow_forwardGive the structure corresponding to each IUPAC name. a. 3-methylhexane c. 3,5,5-trimethyloctane b. 3,3-dimethylpentane d. 3-ethyl-4-methylhexanearrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





