
Concept explainers
Write Lewis symbols for the following atoms. (a) Kr; (b) Ge; (c) N; (d) Ga; (e) As; (f) Rb.
(a)
Interpretation:
Interpret Lewis symbol of Kr.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of Kr is:
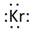
Explanation of Solution
In periodic table, Krypton is present in 18 group and in 4 period. The symbol of Krypton is Kr carrying 8 valence electrons in its valence shell.
Thus, the Lewis symbol of Kr is:
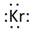
(b)
Interpretation:
Interpret the Lewis symbol of Ge.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of Ge is:

Explanation of Solution
In periodic table, Germanium is present in 14 group and in 4 period. The symbol of Germanium is Ge carrying 4 valence electrons in its valence shell.
Thus, the Lewis symbol of Ge is:

(c)
Interpretation:
Interpret the Lewis symbol of N.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of N is:
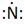
Explanation of Solution
In periodic table, Nitrogen is present in 15 group and in 2 period. The symbol of Nitrogen is N carrying 5 valence electrons in its valence shell.
Thus, the Lewis symbol of N is:
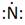
(d)
Interpretation:
Interpret the Lewis symbol of Ga.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of Ga is:
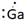
Explanation of Solution
In periodic table, Gallium is present in 13 group and in 4 period. The symbol of Gallium is Ga carrying 3 valence electrons in its valence shell.
Thus, Lewis symbol of Ga is:
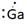
(e)
Interpretation:
Interpret the Lewis symbol of As.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of As is:
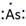
Explanation of Solution
In periodic table, Arsenic is present in 15 group and in 4 period. The symbol of Arsenic is As carrying 5 valence electrons in its valence shell.
Thus, the Lewis symbol of As is:
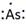
(f)
Interpretation:
Interpret the Lewis symbol of Rb.
Concept introduction:
Lewis symbol is the representation of the atoms in elemental form found in the periodic table. The element is represented with the universal symbol along with the valence electron present in its valence shell.
The valence electron present in the atom is decided by total number of electrons present in outermost shell.
Answer to Problem 1E
Lewis symbol of Rb is:
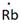
Explanation of Solution
In periodic table, Rubidium is present in 1 group and in 5th period. The symbol of rubidium is Rb carrying 1 valence electron in its valence shell.
Thus, the Lewis symbol of Rb is:
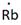
Want to see more full solutions like this?
Chapter 10 Solutions
Mastering Chemistry With Pearson Etext -- Standalone Access Card -- For General Chemistry: Principles And Modern Applications (11th Edition)
Additional Science Textbook Solutions
Inorganic Chemistry
Organic Chemistry (8th Edition)
Chemistry & Chemical Reactivity
Chemistry: Structure and Properties (2nd Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry: The Central Science (14th Edition)
- Write Lewis structures for reactants and products and show by the use of curved arrows how each reaction occursarrow_forwardWhich of the Lewis structures for NO is dominantly based on analysis of the formal charges?arrow_forwardWrite a Lewis structure for the NO3 - ion. Include resonance structures?arrow_forward
- Assign the formal charges of the “heavy” atoms (non-hydrogen atoms, so in this case C’s, N, O and P) in the Lewis structure below. Note that you should not add or erase the lone pairsarrow_forwardHow many covalent bonds are there in ClO2? Assume Cl will expand its octet to minimize formal charges.arrow_forwardWrite a Lewis structure for the amide ion, NH2─, and assign formal charges to each atom.arrow_forward
- How many lone pairs are there on the central Br atom in the Lewis structure of BrF2-?arrow_forwardhow do I solve this problem? How many different types of resonance structures can be drawn for the ion SO32 - where all atoms satisfy the octet rule?arrow_forwardWrite the Lewis symbol for atoms of each of the following elements: a) Te, b) Si, c) Kr, d) P.arrow_forward
- Can someone expain what it means when it says: draw a better resonance structure than the one shown below" does it mean it wants the highly electronegative atoms O and N to be nuetral? they are "happier neutral? do i draw lewis structure variations and then...?arrow_forwardWhat's the written lewis structure for C2H5O2N and how many valence electrons?arrow_forwardBelow are two different Lewis structures for nitrous acid (HNO2). Which is the better Lewis structure based only on formal charge?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

