
Concept explainers
Which pair of elements has the most similar Lewis structures?
a. N and S
b. F and Ar
c. Cl and Ar
d. O and S
Interpretation: The pair of elements that would have the most similar structure is to be determined.
Concept Introduction:
Lewis structure is used to represent the molecules. It is also known as dot structure. In Lewis model, valence electrons are represented as dots.
Answer to Problem 1SAQ
Correct Answer: The most similar Lewis structure is of
Therefore, the correct answer is option (d).
Explanation of Solution
Reason for correct option:
Oxygen
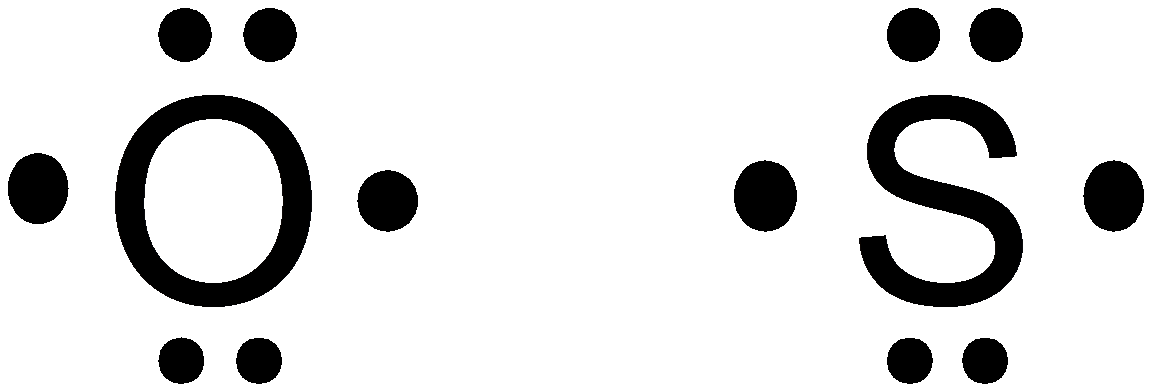
Thus,
Hence, option (d) is correct.
Reasons for incorrect options:
Option (a) is given as
Nitrogen falls in group
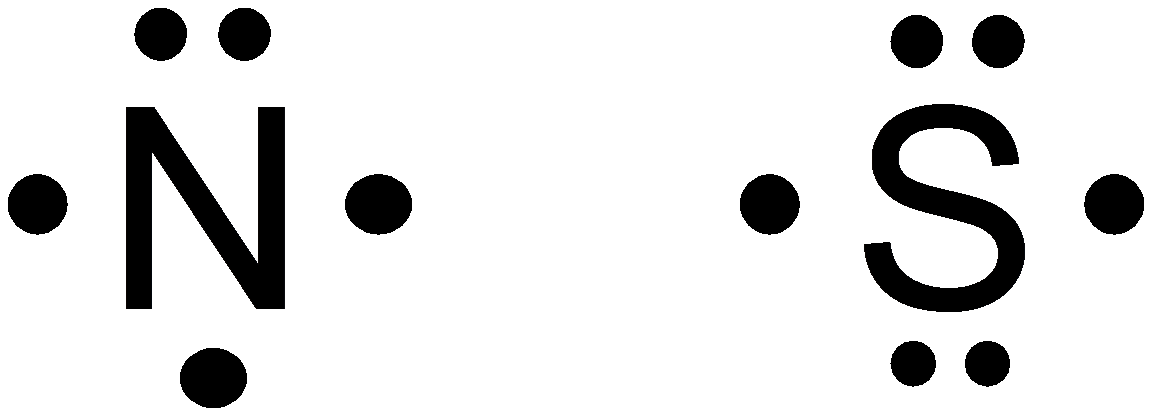
So, it is a wrong answer.
Option (b) is given as
Fluorine falls in
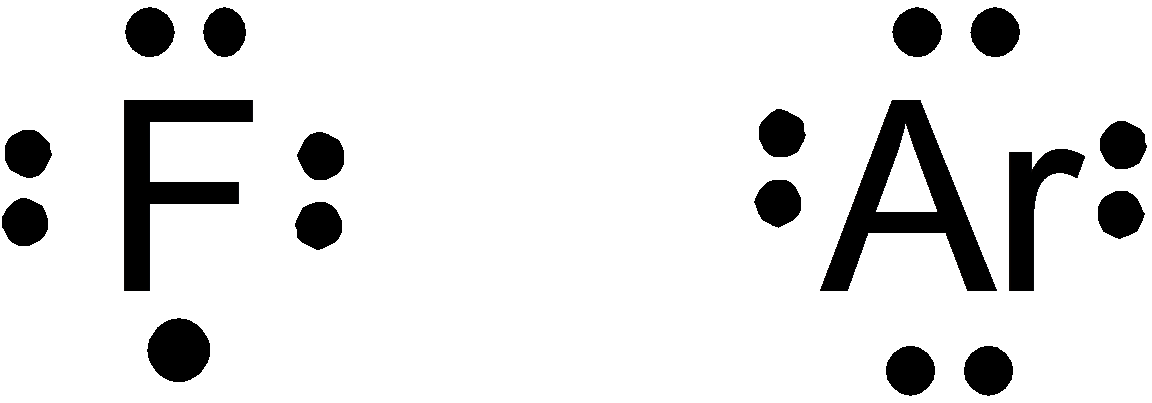
So, it is a wrong answer.
Option (c) is given as
Chlorine falls in group

So, it is a wrong answer.
Hence, options (a), (b) and (c) are incorrect.
Want to see more full solutions like this?
Chapter 10 Solutions
INTRO CHEMISTRY PKG
- Write Lewis structures for the following: (a) ClF3 (b) PCl5 (c) BF3 (d) PF6arrow_forwardCompare your answers from parts a and b of Exercise 69 with H values calculated for each reaction using standard enthalpies of formation in Appendix 4. Do enthalpy changes calculated from bond energies give a reasonable estimate of the actual values?arrow_forwardWrite a generic Lewis structure for the elements in the oxygen family (Group 6A). Do the elements in the oxygen family tend to gain or lose electrons in chemical reactions? How many?arrow_forward
- Write one Lewis structure for it NNO (the atoms are connected in this order)arrow_forwardWhich is true of Lewis structures? a. They do not show details regarding the shape of molecules. b. They show details regarding the shape of molecules.arrow_forwardWrite a generic Lewis structure for the halogens. Do thehalogens tend to gain or lose electrons in chemical reactions?How many?arrow_forward
- Use Lewis structures to explain why Br3 - and I3- are stable, while F3 - is not.arrow_forwardReview the rules for drawing Lewis structures and try to draw the correct Lewis structures for the molecules shown in part A of this exercise.arrow_forwardThe Lewis structure shows the number of ______ electrons in an atom. Only______electrons are placed on each side of the chemical symbol. a. bonding, eight b. nonbonding, eightc. valence, one d. valence, twoarrow_forward
- Is this a valid Lewis structure?arrow_forwardThese Lewis structures are incorrect. Explain what is wrong in each one and give the correct structure for the molecule.arrow_forwardThe Lewis structure for OCl₂ shown below is incorrect. Starting from this structure, complete the correct structure.arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





