
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 71QAP
One mole of Na2S is represented as  where
where  represents Na and
represents Na and  represents S. Complete the picture showing only the sodium and sulfide ions. The water molecules need not be shown.
represents S. Complete the picture showing only the sodium and sulfide ions. The water molecules need not be shown.
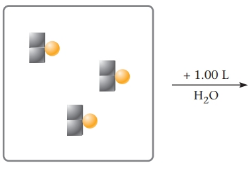
What is the molarity of Na+? of S2-?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Chemistry: Principles and Reactions
Ch. 10 - A solution is prepared by dissolving 12.15 g of...Ch. 10 - Prob. 2QAPCh. 10 - Prob. 3QAPCh. 10 - Solutions Introduced directly into the bloodstream...Ch. 10 - Silver ions can be found in some of the city water...Ch. 10 - Prob. 6QAPCh. 10 - Complete the following table for aqueous solutions...Ch. 10 - Complete the following table for aqueous solutions...Ch. 10 - Complete the following table for aqueous solutions...Ch. 10 - Complete the following table for aqueous solutions...
Ch. 10 - Prob. 11QAPCh. 10 - Prob. 12QAPCh. 10 - Prob. 13QAPCh. 10 - A solution is prepared by diluting 0.7850 L of...Ch. 10 - A bottle of phosphoric acid is labeled 85.0% H3PO4...Ch. 10 - Prob. 16QAPCh. 10 - Complete the following table for aqueous solutions...Ch. 10 - Complete the following table for aqueous solutions...Ch. 10 - Assume that 30 L of maple sap yields one kilogram...Ch. 10 - Juice (d=1.0g/mL) from freshly harvested grapes...Ch. 10 - Prob. 21QAPCh. 10 - Which of the following is more likely to be...Ch. 10 - Prob. 23QAPCh. 10 - Prob. 24QAPCh. 10 - Consider the process by which lead chloride...Ch. 10 - Prob. 26QAPCh. 10 - The Henry's law constant for the solubility of...Ch. 10 - The Henry's law constant for the solubility of...Ch. 10 - A carbonated beverage is made by saturating water...Ch. 10 - Air contains 78% nitrogen. At 25C, Henry's law...Ch. 10 - Vodka is advertised to be 80 proof. That means...Ch. 10 - What is the freezing point of maple syrup (66%...Ch. 10 - Calculate the vapor pressure of water over each of...Ch. 10 - Calculate the vapor pressure of water over each of...Ch. 10 - Prob. 35QAPCh. 10 - Consider an aqueous solution of urea, (CO(NH2)2)...Ch. 10 - Prob. 37QAPCh. 10 - Prob. 38QAPCh. 10 - Calculate the freezing point and normal boiling...Ch. 10 - How many grams of the following nonelectrolytes...Ch. 10 - What is the freezing point and normal boiling...Ch. 10 - Antifreeze solutions are aqueous solutions of...Ch. 10 - When 13.66 g of lactic acid, C3H6O3, are mixed...Ch. 10 - A solution consisting of 4.50 g of propylene...Ch. 10 - Insulin is a hormone responsible for the...Ch. 10 - Epinephrine (or adrenaline) is a hormone and...Ch. 10 - Lauryl alcohol is obtained from the coconut and is...Ch. 10 - The Rast method uses camphor (C10H16O) as a...Ch. 10 - Caffeine is made up of 49.5% C, 5.2% H, 16.5% O,...Ch. 10 - A compound contains 42.9% C, 2.4% H, 16.6% N, and...Ch. 10 - A biochemist isolates a new protein and determines...Ch. 10 - Prob. 52QAPCh. 10 - Estimate the freezing and boiling points of normal...Ch. 10 - Arrange 0.10 m aqueous solutions of the following...Ch. 10 - Aqueous solutions introduced into the stream y...Ch. 10 - What is the osmotic pressure of a 0.135 M solution...Ch. 10 - The freezing point of a 0.11 m solution of HNO2 is...Ch. 10 - The freezing point of a 0.21 m aqueous solution of...Ch. 10 - An aqueous solution of LiX is prepared by...Ch. 10 - An aqueous solution of M2O is prepared by...Ch. 10 - A sucrose (C12H22O11) solution that is 45.0%...Ch. 10 - An aqueous solution made up of 32.47 g of...Ch. 10 - How would you prepare 5.00 L of a solution that is...Ch. 10 - Carbon tetrachloride (CCl4) boils at 76.8C and has...Ch. 10 - Twenty-five milliliters of a solution...Ch. 10 - The Henry's law constant for the solubility of...Ch. 10 - Prob. 67QAPCh. 10 - Consider two solutions at a certain temperature....Ch. 10 - A pharmacist prepares an isotonic saline solution...Ch. 10 - One mole of CaCl2 is represented as where...Ch. 10 - One mole of Na2S is represented as where...Ch. 10 - Prob. 72QAPCh. 10 - Consider three test tubes. Tube A has pure water....Ch. 10 - The freezing point of 0.20 m HF is -0.38C. Is HF...Ch. 10 - A certain gaseous solute dissolves in water,...Ch. 10 - The freezing point of 0.10 M KHSO3 is -0.38C....Ch. 10 - Consider 2 vapor pressure curves A and B. They are...Ch. 10 - A gaseous solute dissolves in water. The solution...Ch. 10 - In your own words, explain (a) why seawater has a...Ch. 10 - Prob. 80QAPCh. 10 - Beaker A has 1.00 mol of chloroform, CHCl3, at...Ch. 10 - Prob. 82QAPCh. 10 - Prob. 83QAPCh. 10 - Prob. 84QAPCh. 10 - Prob. 85QAPCh. 10 - A martini, weighing about 5.0 oz (142 g), contains...Ch. 10 - When water is added to a mixture of aluminum metal...Ch. 10 - Prob. 88QAPCh. 10 - Prob. 89QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One mole of CaCl2 is represented as where represents Ca and represents Cl. Complete the picture showing only the calcium and chloride ions. The water molecules need not be shown. What is the molarity of Ca2+? of Cl-?arrow_forwardStock solutions of HCl with various molarities are frequentlyprepared. Complete Table 14.7 by calculatingthe volume of concentrated, or 12M, hydrochloric acidthat should be used to make 1.0 L of HCl solution witheach molarity listed.arrow_forwardWhich of the principal characteristics of solutions can we see in the solutions of K2Cr2O7 shown in Figure 11.2?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY