
The amount of weight percent sulfur.
Answer to Problem 79AAP
The amount of weight percent sulfur is
Explanation of Solution
Show the chemical structure bonding of polybutadiene and polystyrene as:
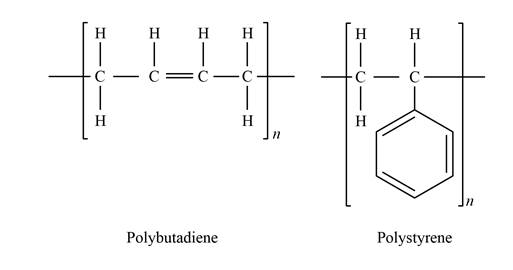
Express the weight per cent of sulfur.
Here, the molar mass of the sulfur is
Calculate the mass of sulfur at 20%.
Here, the number of mole in butadiene is
Calculate the copolymer total mass.
Here, the molar mass of the polybutadlene is
Conclusion:
The average molecular mass of the polylstyrene is
The average molecular mass of the polybutadlene is
The average molecular mass of the sulfur is
Substitute
Calculate the fraction of butadlene.
Calculate the number of moles of butadlene using 100 g of copolymer.
Substitute 1.45 g for mole butadlene and
Substitute 9.28 g for
Thus, the amount of weight percent sulfur is
Want to see more full solutions like this?
Chapter 10 Solutions
Foundations of Materials Science and Engineering
- :. Differentiate between Thermoplastics and Thermosets plastics. Explain in detail the uses of these plastics in the world.arrow_forwardWhy do thermoplastic polymers start to turn white as they are plastically deformed?arrow_forwardDraw the structure of the idealized modulus-temperature for thermoplastic and thermosetscurveand mention all the transitions.arrow_forward
- What is ceiling temperature in thermodynamics of polymerization? Explain the correlation of ceiling temperature with polymers. Show the equations to support your answer.arrow_forwardDefine the term Elastoplastic?arrow_forwardWhat are the two micro-scale plasticity mechanisms active in amorphous polymers? Are they likely to occur simultaneously? Explain.arrow_forward
- Bakelite is one of the widely ___________________ polymer. Select one: Thermoset Elastomers Composite Thermoplasticarrow_forwardWhat polymer derived from acetylene gas is called polychloroprene (neoprene)?arrow_forwardExplain how macroscopic plasticity occurs in polymers from the point of view of microscopic mechanisms (covered in the chapters indicated in the Callister book). (maximum of 3 lines, not counting the space for possible schematic figures).arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





