
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.SE, Problem 18MP
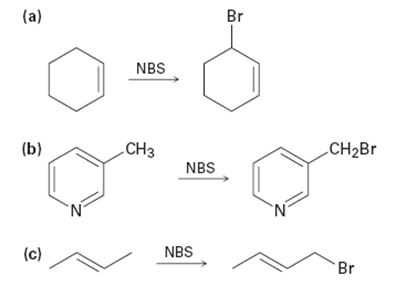
Draw the electron-pushing mechanism for the propagation steps of the allylic bromination reactions below. You may omit NBS in your mechanism, and use Br ∙ and Br2.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Complete the reactions given below, write down the type of mechanism (SN1, SN2, E1, E2)?
is this an E1 or E2 mechanism for this reaction? What is the major product and mechanism for it?
Draw the product of an SN2 reaction shown below. Use wedge and dash bonds to indicate stereochemistry where appropriate. Ignore inorganic byproducts.
Chapter 10 Solutions
Organic Chemistry
Ch. 10.1 - Prob. 1PCh. 10.1 - Draw structures corresponding to the following...Ch. 10.2 - Prob. 3PCh. 10.2 - Taking the relative reactivities of 1°, 2°, and...Ch. 10.4 - Prob. 5PCh. 10.4 - The major product of the reaction of...Ch. 10.4 - Prob. 7PCh. 10.5 - Prob. 8PCh. 10.6 - Prob. 9PCh. 10.6 - How might you replace a halogen substituent by a...
Ch. 10.7 - How would you carry out the following...Ch. 10.8 - Rank both sets of compounds in order of increasing...Ch. 10.8 - Tell whether each of the following reactions is an...Ch. 10.SE - Prob. 14VCCh. 10.SE - Prob. 15VCCh. 10.SE - Prob. 16VCCh. 10.SE - Draw the electron-pushing mechanism for each...Ch. 10.SE - Draw the electron-pushing mechanism for the...Ch. 10.SE - The formation of Br2 from NBS first involves the...Ch. 10.SE - In light of the fact that tertiary alkyl halides...Ch. 10.SE - Alkyl halides can be reduced to alkanes by a...Ch. 10.SE - Name the following alkyl halides:Ch. 10.SE - Prob. 23APCh. 10.SE - Draw and name all of the monochlorination products...Ch. 10.SE - How would you prepare the following compounds,...Ch. 10.SE - Prob. 26APCh. 10.SE - A chemist requires a large amount of...Ch. 10.SE - What product(s) would you expect from the reaction...Ch. 10.SE - What product(s) would you expect from the reaction...Ch. 10.SE - What product would you expect from the reaction of...Ch. 10.SE - Rank the compounds in each of the following series...Ch. 10.SE - Which of the following compounds have the same...Ch. 10.SE - Tell whether each of the following reactions is an...Ch. 10.SE - Prob. 34APCh. 10.SE - Alkylbenzenes such as toluene (methylbenzene)...Ch. 10.SE - Prob. 36APCh. 10.SE - Prob. 37APCh. 10.SE - Prob. 38APCh. 10.SE - Prob. 39APCh. 10.SE - Prob. 40APCh. 10.SE - The syntheses shown here are unlikely to occur as...Ch. 10.SE - Why do you suppose its not possible to prepare a...Ch. 10.SE - Prob. 43APCh. 10.SE - Identify the reagents a–c in the following...Ch. 10.SE - Prob. 45APCh. 10.SE - Prob. 46APCh. 10.SE - Prob. 47APCh. 10.SE - The relative rate of radical bromination is...Ch. 10.SE - Prob. 49APCh. 10.SE - Predict the product and provide the entire...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the product of an SN2 reaction shown below. Use wedge and dash bonds to indicate stereochemistry where appropriate. Indicate inorganic byproducts.arrow_forwardA student proposes the following reaction mechanism for the reaction in Model 6. Which step inthis mechanism is least favorable? Explain your reasoning.arrow_forwardIn the box to the left of each reaction below, write the mechanism by which it occurs (could be SN1, SN2, or E1, or even 2 of them). Then draw the product(s).arrow_forward
- Draw the reaction mechanism that shows BH3 adding to the alkene. Why is the product anti-Markovnikov?arrow_forwardWhat happens to the rate of an SN1 reaction under the following conditions? [RX] is halved, and [:Nu−] stays the samearrow_forward"Drawing the Product of Inversion in an SN2 Reaction Label the nucleophile and leaving group, and draw the product (including stereochemistry) of the following SN2 reaction.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License