Barbiturates are prepared by treating a derivative of diethyl malonate with urea in the presence of sodium ethoxide as a catalyst. Following is an equation for the preparation of barbital, a long-duration hypnotic and sedative, from diethyl diethylmalonate and urea. -OEt H,N -NH 1. EtO-Na+ + 2 ELOH 2. Н,О -OEt H,N -NH Diethyl diethylmalonate Urea 5,5-Diethylbarbituric acid (Barbital) Barbital is prescribed under one of a dozen or more trade names.
Barbiturates are prepared by treating a derivative of diethyl malonate with urea in the presence of sodium ethoxide as a catalyst. Following is an equation for the preparation of barbital, a long-duration hypnotic and sedative, from diethyl diethylmalonate and urea. -OEt H,N -NH 1. EtO-Na+ + 2 ELOH 2. Н,О -OEt H,N -NH Diethyl diethylmalonate Urea 5,5-Diethylbarbituric acid (Barbital) Barbital is prescribed under one of a dozen or more trade names.
Chapter23: Carbonyl Condensation Reactions
Section23.SE: Something Extra
Problem 45MP
Related questions
Question
Propose a mechanism for this reaction
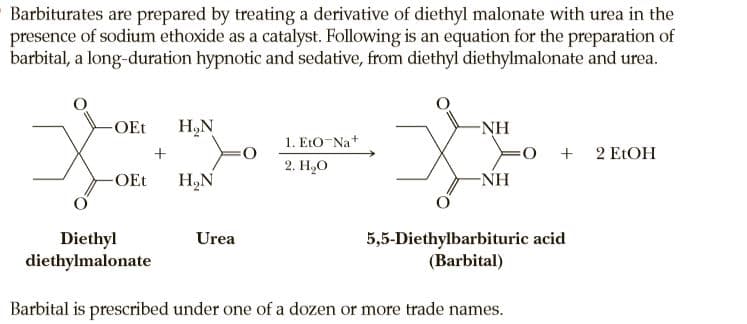
Transcribed Image Text:Barbiturates are prepared by treating a derivative of diethyl malonate with urea in the
presence of sodium ethoxide as a catalyst. Following is an equation for the preparation of
barbital, a long-duration hypnotic and sedative, from diethyl diethylmalonate and urea.
-OEt
H,N
-NH
1. EtO-Na+
+ 2 ELOH
2. Н,О
-OEt
H,N
-NH
Diethyl
diethylmalonate
Urea
5,5-Diethylbarbituric acid
(Barbital)
Barbital is prescribed under one of a dozen or more trade names.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning