
Experimental data are listed here for the reaction ![Chapter 11, Problem 11.20PAE, Experimental data are listed here for the reaction B: Time (s) IB] (mol/L) 0.00 0.000 10.0 0.326](https://content.bartleby.com/tbms-images/9781337398909/Chapter-11/images/html_98909-11-11.20pae_image001.jpg) B:
B:
| Time (s) | IB] (mol/L) |
| 0.00 | 0.000 |
| 10.0 | 0.326 |
| 20.0 | 0.572 |
| 30.0 | 0.750 |
| 40.0 | 0.890 |
- Prepare a graph from these data, connect the points with a smooth line, and calculate the rate of change of [B] for each 10-s interval from 0.0 to 40.0 s. Does the rate of change decrease from one time interval to the next? Suggest a reason for this result.
- How is the rate of change of [AJ related to the rate of change of [B] in each time interval? Calculate the rate of change of [AJ for the time interval from 10.0 to 20.0 s.
- What is the instantaneous rate, A[B]/Ar, when [BI = 0.750 mol/L?
a)
Interpretation:
A graph based on the given data must be plotted and the rate of change of [B] for every 10 s interval must be calculated.
Concept Introduction:
- Chemical reactions proceed at a certain rate which is represented in terms of the change in concentration over a certain period of time
- The rate can be expressed either in terms of a decrease in concentration of the reactants or an increase in the concentration of products.
Answer to Problem 11.20PAE
Solution:
The plot is depicted below.
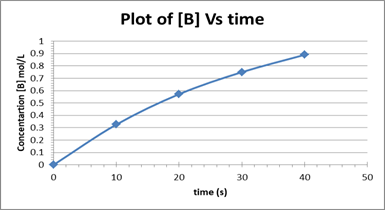
The rate of change of [B] decreases from one-time interval to the next.
Explanation of Solution
The given reaction is:
A plot of concentration of [B] vs time based on the given data is shown below:
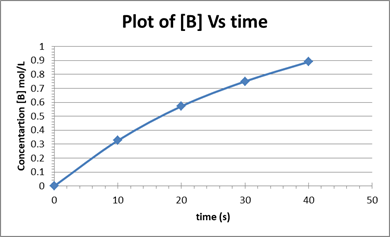
As per the above calculations, the rate of change decreases from one-time interval to the next. This is because as time increases the concentration of reactants decreases as a result the rate of formation of the products will also decrease.
(b)
Interpretation:
The relation between the rate of change of [A] and the rate of change of [B] must be explained. The rate of change of [A] for the time interval from 10.0 to 20.0 s should be calculated.
Concept Introduction:
- Chemical reactions proceed at a certain rate which is represented in terms of the change in concentration over a certain period of time
- The rate can be expressed either in terms of a decrease in concentration of the reactants or an increase in the concentration of products.
Answer to Problem 11.20PAE
Solution:
The rate of change of A is half that of B and for time interval 10 to 20 s rate of change of [A] is
Explanation of Solution
The given reaction is:
Based on the stoichiometry of this reaction, the rate can be expressed as:
For the time interval between t = 10 to t = 20 s:
(c)
Interpretation:
The instantaneous rate must be calculated when [B] = 0.750 mol/L
Concept Introduction:
- Chemical reactions proceed at a certain rate which is represented in terms of the change in concentration over a certain period of time
- The rate can be expressed either in terms of a decrease in concentration of the reactants or an increase in the concentration of products.
- Average rate of a reaction can be defined as the difference in the concentrations measured at two different times whereas, instantaneous rate can be defined as the rate of a reaction at a particular instant in time.
Answer to Problem 11.20PAE
Solution: Rate = 0.200 mol/L-s
Explanation of Solution
Instantaneous rate can be deduced by drawing a tangent at the point of the curve that corresponds to a particular instant. The slope of the tangent gives the instantaneous rate. In this case the value of [B] = 0.075 mol/L corresponds to a time t = 30 sec. The tangent and the slope are depicted in the plot shown below:
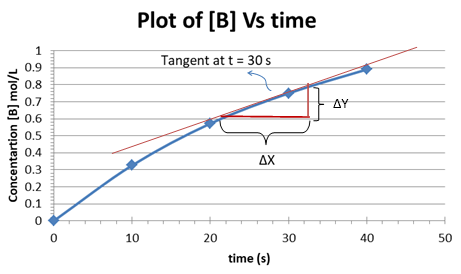
Therefore, the instantaneous rate is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Engineering Students
- Experimental data are listed here for the reaction A 2 B. (a) Prepare a graph from these data; connect the points with a smooth line; and calculate the rate of change of [B] for each 10-second interval from 0.0 to 40.0 seconds. Does the rate of change decrease from one time interval to the next? Suggest a reason for this result. (b) How is the rate of change of [A] related to the rate of change of [B] in each time interval? Calculate the rate of change of [A] for the time interval from 10.0 to 20.0 seconds.arrow_forwardThe reaction 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g) was studied at 904 C, and the data in the table were collected. (a) Determine the order of the reaction for each reactant. (b) Write the rate equation for the reaction. (c) Calculate the rate constant for the reaction. (d) Find the rate of appearance of N2 at the instant when [NO] = 0.350 mol/L and [H] = 0.205 mol/L.arrow_forwardA study of the rate of the reaction represented as 2AB gave the following data: Time (s) 0.0 5.0 10.0 15.0 20.0 25.0 35.0 [A](M) 1.00 0.775 0.625 0.465 0.350 0.205 0.230 (a) Determine the average rate of disappearance of A between 0.0 s and 10.0 s, and between 10.0 s and 20.0 s. (b) Estimate the instantaneous rate of disappearance of A at 15.0 s from a graph of time versus [A]. What are theunits of this rate? (c) Use the rates found in parts (a) and (b) to determine the average rate of formation of B between 0.00 s and 10.0 s, and the instantaneous rate of formation of B at 15.0 s.arrow_forward
- At 40C, H2O2 (aq) will decompose according to the following reaction: 2H2O2(aq)2H2O(l)+O2(g) The following data were collected for the concentration of H2O2 at various times. Times(s) [H2O2](mol/L) 0 1.000 2.16 104 0.500 4.32 104 0.250 a. Calculate the average rate of decomposition of H2O2 between 0 and 2.16 104 s. Use this rate to calculate the average rate of production of O2(g) over the same time period. b. What are these rates for the time period 2.16 104 s to 4.32 104 s?arrow_forwardConsider the decomposition reaction 2X2Y+ZThe following graph shows the change in concentration with respect to time for the reaction. What does each of the curves labeled 1, 2, and 3 represent?arrow_forwardBased on the kinetic theory of matter, what would the action of a catalyst do to a reaction that is the reverse of some reaction that we say is catalyzed?arrow_forward
- Candle wax is a mixture of hydrocarbons. In the reaction of oxygen with candle w ax in Figure 11.2, the rate of consumption of oxygen decreased with time after the flask was covered, and eventually' the flame went out. From the perspective of the kinetic-molecular theory, describe what is happening in the flask. FIGURE 11.2 When a candle burns in a closed container, the flame will diminish and eventually go out. As the amount of oxygen present decreases, the rate of combustion will also decrease. Eventually, the rate of combustion is no longer sufficient to sustain the flame even though there is still some oxygen present in the vessel.arrow_forwardChlorine dioxide, ClO2, is a reddish-yellow gas that is soluble in water. In basic solution it gives ClO3 and ClO2 ions. 2ClO2(aq)+2OH(aq)ClO3(aq)+ClO2(aq)+H2O To obtain the rate law for this reaction, the following experiments were run and, for each, the initial rate of reaction of ClO2 was determined. Obtain the rate law and the value of the rate constant.arrow_forwardA study of the rate of dimerization of C4H6 gave the data shown in the table: 2C4H6C8H12 (a) Determine the average rate of dimerization between 0 s and 1600 s, and between 1600 s and 3200 s. (b) Estimate the instantaneous rate of dimerization at 3200 s from a graph of time versus [C4H6]. What are the units of this rate? (c) Determine the average rate of formation of C8H12 at 1600 s and the instantaneous rate of formation at 3200 s from the rates found in parts (a) and (b).arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





