
Concept explainers
Consider a substance X with a ΔHvap = 20.3 kJ/mol and ΔHfus = 9.0 kJ/mol. The melting point, freezing point, and heat capacities of both the solid and liquid X are identical to those of water.
- a If you place one beaker containing 50 g of X at −10°C and another beaker with 50 g of H2O at −10°C on a hot plate and start heating them, which material will reach the boiling point first?
- b Which of the materials from part a, X or H2O, would completely boil away first?
- c On a piece of graph paper, draw the heating curve for H2O and X. How do the heating curves reflect your answers from parts a and b?
(a)
Interpretation:
The substance X with
Explanation of Solution
Explanation
To explain: which material will attain the boiling point earlier when heating
Comparing the given values with water
We should need to compare the vaporization of the given substance and heat of fusion with the values of water. For water,
Heating the substance or water from
In the first step, solid has heated from
Where,
In second step, the solid is melted to liquid at
Where,
In third step, the liquid has heated from
Where,
The heat capacity, temperature change and mass of the substance X is identical to water, the heat required for first and third step are same for both. As the heat of fusion is higher for substance X and in step two, heat required for substance X take longer time. So, water will reach the boiling point earlier than substance X.
(b)
Interpretation:
The substance X with
Explanation of Solution
Explanation
To identify: the material which is totally boiled first from part (a)
Comparing the given values with water
We should need to compare the vaporization of the given substance and heat of fusion with the values of water. For water,
Heating the substance or water from
In the first step, solid has heated from
Where,
In second step, the solid is melted to liquid at
Where,
In third step, the liquid has heated from
Where,
To entirely boil away the substance a further step is needed (step 4).
In fourth step, liquid is boiled to vapour at
As the values of heat of vaporization is much greater than the heat of fusion values. In this step needed much more heat than in step 2 for both water and substance X. Since, heat of vaporisation is less for substance X per mole. Fourth step will require small heat for X and hence it will take less time. The total heat required for fourth step is directly proportional to the time taken for entirely boil away the substance X. However forth step require more time to complete this step. Hence, substance X will boil way first.
(c)
Interpretation:
The substance X with
Explanation of Solution
Explanation
To draw: heating curve for both water and substance X.
Comparing the given values with water
We should need to compare the vaporization of the given substance and heat of fusion with the values of water. For water,
Heating the substance or water from
In the first step, solid has heated from
Where,
In second step, the solid is melted to liquid at
Where,
In third step, the liquid has heated from
Where,
The heating curve for both water and substance X are illustrated below.
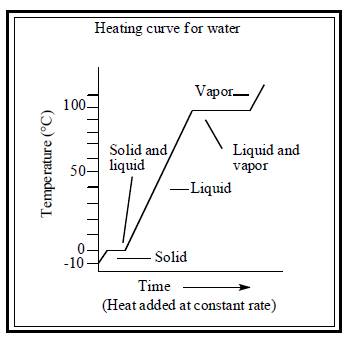
Figure 1
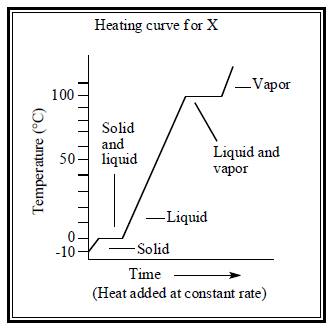
Figure 2
Want to see more full solutions like this?
Chapter 11 Solutions
General Chemistry - Standalone book (MindTap Course List)
- Why are steam burns so much worse than water burns even if the H2O is at the same temperature for both phases? Hint: Consider the heat of vaporization of water.arrow_forwardWhat mass (g) of ethanol, CH3CH2OH(), can be vaporized at its boiling point of 78.4 C by transfer of 500. kJ to the liquid? The vapH of ethanol is 38.6 kJ/mol at this temperature.arrow_forwardWhy does sweating cool the human body?arrow_forward
- A 1.40-g sample of polyethylene, a common plastic, is dissolved in enough organic solvent to give 100.0 mL of solution. What is the average molar mass of the polymer if the measured osmotic pressure of the solution is 1.86 mm Hg at 25 C?arrow_forwardThe cooling effect of alcohol on the skin is due to its evaporation. Calculate the heat of vaporization of ethanol (ethyl alcohol), C2H5OH. C2H5OH(l)C2H5OH(g);H=? The standard enthalpy of formation of C2H5OH(l) is 277.7 kJ/mol and that of C2H5OH(g) is 235.1 kJ/mol.arrow_forwardThe enthalpy of vaporization of water is larger than its enthalpy of fusion. Explain why.arrow_forward
- What is the enthalpy change when a 1.00-kg block of dry ice, CO2(s), sublimes at 78 C? The enthalpy of sublimation of CO2(s) is 26.9 kJ/mol. Is this process exothermic or endothermic?arrow_forwardMethane, CH4, reacts with chlorine, Cl2, to produce a series of chlorinated hydrocarbons: methyl chloride (CH3Cl), methylene chloride (CH2Cl3), chloroform (CHCl3), and carbon tetrachloride (CCl4). Which compound has the highest vapor pressure at room temperature? Explain.arrow_forwardAre changes in state physical or chemical changes? Explain. What type of forces must be overcome to melt or vaporize a substance (are these forces intramolecular or intermolecular)? Define the molar heat of fusion and molar heat of vaporization. Why is the molar heat of vaporization of water so much larger than its molar heat of fusion? Why does the boiling point of a liquid vary with altitude?arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





